In the field of respiratory care, understanding a patient’s acid-base status is vital. One essential value that helps assess this status is base excess (BE). Often included in arterial blood gas (ABG) reports, BE reflects the metabolic component of the body’s acid-base balance and provides critical insights into whether a patient is experiencing a metabolic acidosis or alkalosis.
For respiratory therapists, BE is more than just a number—it is a powerful tool that aids in the diagnosis and management of a wide variety of respiratory and metabolic conditions. This article explains what base excess is, how it is interpreted, and why it matters in clinical practice.
Take our free course to master the basics of ABG interpretation with clear explanations and helpful practice questions.
What is Base Excess?
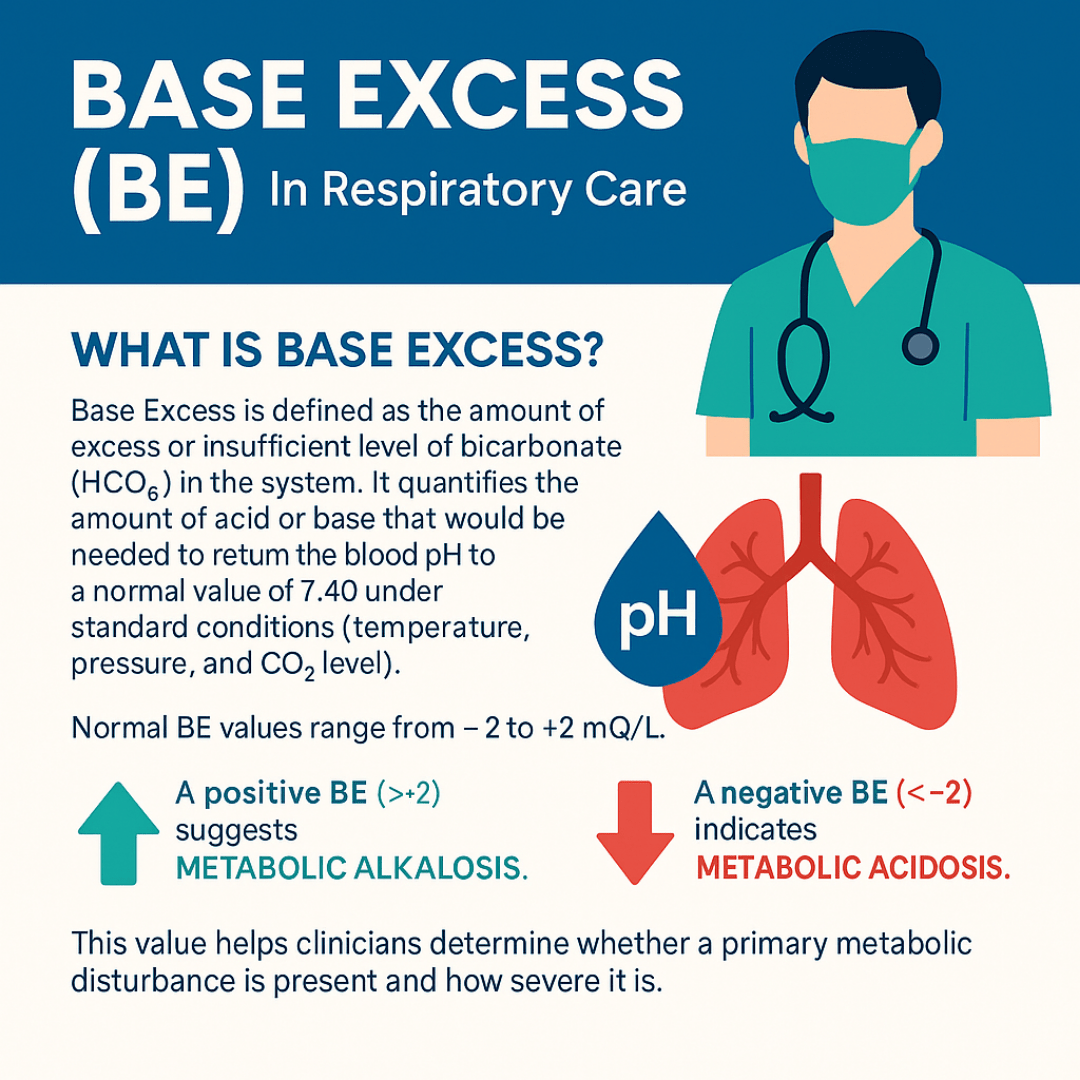
Base excess (BE) is defined as the amount of excess or insufficient level of bicarbonate (HCO₃⁻) in the system. It quantifies the amount of acid or base that would be needed to return the blood pH to a normal value of 7.40 under standard conditions (temperature, pressure, and CO₂ level).
In simpler terms, BE measures the metabolic (non-respiratory) contribution to acid-base balance. It isolates the role of the kidneys and other metabolic systems by removing the effect of CO₂, which is controlled by the lungs.
Normal Values
A normal BE typically ranges from -2 to +2 mEq/L.
- A positive BE (> +2) suggests metabolic alkalosis.
- A negative BE (< -2) indicates metabolic acidosis.
Note: This value helps clinicians determine whether a primary metabolic disturbance is present and how severe it is.
How is Base Excess Calculated?
Base excess is not directly measured but is calculated using complex equations that factor in:
- pH
- PaCO₂ (partial pressure of carbon dioxide)
- Hemoglobin concentration
- Bicarbonate (HCO₃⁻)
Modern blood gas analyzers typically provide BE automatically alongside other ABG parameters such as pH, PaCO₂, PaO₂, and HCO₃⁻.
There are two common types:
- Actual Base Excess (ABE): Uses current hemoglobin levels and other variables.
- Standard Base Excess (SBE): Adjusts BE to standard conditions to eliminate variability, making it more universally comparable.
Why Base Excess Matters in Respiratory Care
Base Excess is particularly relevant in respiratory care for several reasons:
1. Evaluating Metabolic Disorders
Respiratory therapists often manage patients with complex acid-base disturbances. BE helps distinguish whether an abnormal pH is due to a metabolic issue, a respiratory issue, or a combination of both.
For example:
- A low pH and low BE indicate metabolic acidosis, possibly from conditions like diabetic ketoacidosis or renal failure.
- A high pH and high BE suggest metabolic alkalosis, which may result from excessive vomiting or diuretic use.
2. Identifying Compensation
When a primary respiratory problem occurs (e.g., respiratory acidosis from hypoventilation), the kidneys try to compensate by retaining bicarbonate. Base excess helps quantify the effectiveness and completeness of this compensation.
- A normal BE in the presence of respiratory acidosis may mean acute compensation has not yet occurred.
- An elevated BE may indicate chronic compensation, as seen in chronic obstructive pulmonary disease (COPD).
3. Guiding Treatment Decisions
Understanding whether a patient’s acid-base imbalance is respiratory or metabolic helps clinicians tailor interventions:
- In metabolic acidosis, treatment might include administering sodium bicarbonate (in severe cases) or addressing the underlying cause, such as sepsis or kidney dysfunction.
- In metabolic alkalosis, correcting volume depletion or reducing bicarbonate intake may be necessary.
4. Monitoring Critical Illness
In intensive care settings, critically ill patients often experience mixed acid-base disorders. BE can be a helpful marker in tracking the progression or resolution of these issues. For instance:
- A worsening negative BE may indicate shock or worsening metabolic acidosis.
- An improving BE suggests effective therapy and stabilization.
Clinical Examples
Case 1: Metabolic Acidosis
- pH: 7.28
- PaCO₂: 38 mmHg
- HCO₃⁻: 17 mEq/L
- Base Excess: -8 mEq/L
Interpretation: The pH is low, and both HCO₃⁻ and BE are significantly reduced, confirming uncompensated metabolic acidosis.
Case 2: Compensated Respiratory Acidosis
- pH: 7.36
- PaCO₂: 60 mmHg
- HCO₃⁻: 33 mEq/L
- Base Excess: +6 mEq/L
Interpretation: The pH is near normal, but PaCO₂ is high and BE is elevated, suggesting compensated respiratory acidosis, likely in a COPD patient.
Limitations and Considerations
Although BE is a valuable indicator, it should never be interpreted in isolation. A full ABG interpretation always includes reviewing:
- pH (to determine acidemia or alkalemia)
- PaCO₂ (to assess respiratory contribution)
- HCO₃⁻ and BE (for metabolic status)
- Clinical context (history, symptoms, comorbidities)
Note: In conditions involving significant fluid shifts, like sepsis or shock, BE may not reflect total body buffering accurately. It is one piece of the puzzle in a comprehensive assessment.
Base Excess Practice Questions
1. Base excess is defined as the amount of what substance that must be added to fully oxygenated blood to return the pH to 7.40 at 37°C and a pCO₂ of 40 mmHg?
Strong acid
2. A base deficit (or negative base excess) is defined as the amount of what substance that must be added to correct the pH to 7.40?
Strong base
3. What is the difference between actual and standard base excess?
Actual base excess reflects what’s in the blood, while standard base excess accounts for hemoglobin standardized at 5 g/dL to reflect extracellular fluid.
4. A positive base excess value typically indicates what type of acid-base condition?
Metabolic alkalosis
5. A negative base excess value is most indicative of which condition?
Metabolic acidosis
6. What is the typical reference range for base excess in mEq/L?
−2 to +2
7. How is base excess usually reported in laboratory results?
As a concentration in mEq/L
8. What does base excess help determine in acid-base evaluation?
Whether the disturbance is respiratory, metabolic, or mixed
9. While carbon dioxide defines the respiratory component of acid-base balance, what does base excess define?
The metabolic component
10. Base excess is calculated under a standardized pressure of CO₂ by titrating blood back to what pH?
7.40
11. Which buffer in the blood is the primary contributor to base excess?
Bicarbonate
12. What condition is associated with a base excess greater than +2 mEq/L?
Metabolic alkalosis
13. What condition is associated with a base excess less than −2 mEq/L?
Metabolic acidosis
14. What are two causes of a high base excess value?
Compensation for respiratory acidosis and loss of gastric acid via vomiting
15. What lab value helps differentiate between acid addition and bicarbonate loss in a base deficit?
Anion gap
16. A base deficit with an elevated anion gap suggests what underlying issue?
Acid accumulation, such as ketoacidosis
17. A base deficit with a normal anion gap suggests what cause?
Loss of bicarbonate, such as from diarrhea
18. During bicarbonate excretion, what ion is retained to maintain the anion gap?
Chloride
19. What are the two common mechanisms responsible for a base deficit?
Excretion of bicarbonate or neutralization by organic acids
20. Which condition leads to a base deficit due to ketone body accumulation?
Diabetic ketoacidosis
21. What is the main cause of lactic acidosis contributing to a base deficit?
Anaerobic metabolism during hypoxia or strenuous exercise
22. Why does chronic renal failure often lead to a base deficit?
It prevents acid excretion and bicarbonate resorption
23. How does diarrhea lead to a base deficit?
By excessive loss of bicarbonate
24. Which toxic ingestions can cause a base deficit by introducing acid?
Methanol, ethylene glycol, or aspirin overdose
25. What is base excess also known as in clinical terminology?
Buffer base capacity
26. What does a high positive base excess (> +2) strongly indicate?
Presence of a metabolic alkalosis
27. What does a low base excess (more negative than −2) suggest about the acid-base balance?
Metabolic acidosis from insufficient base
28. What compensatory response is expected from the kidneys during negative base excess?
Excretion of hydrogen ions and reabsorption of bicarbonate
29. What is the purpose of increasing bicarbonate reabsorption in metabolic acidosis?
To neutralize excess acid and raise the pH
30. Which three clinical conditions are major causes of negative base excess?
Lactic acidosis, diabetic ketoacidosis, and acute renal failure
31. What are three causes of positive base excess (metabolic alkalosis)?
Vomiting, gastric suction, and excessive IV bicarbonate administration
32. Which types of molecules act as bases by accepting hydrogen ions in the body?
Bicarbonate, phosphate, and protein
33. What is the anion gap, and how is it calculated?
The difference between measured cations (mainly sodium) and anions (chloride and bicarbonate) in serum.
34. What is the normal reference range for the anion gap in mEq/L?
8 to 12 mEq/L
35. What is the clinical significance of an elevated anion gap?
It helps identify the presence and cause of metabolic acidosis.
36. Which cation accounts for over 90% of the circulating serum cations?
Sodium (Na⁺)
37. Which two anions account for about 85% of the total serum anions?
Chloride (Cl⁻) and bicarbonate (HCO₃⁻)
38. What are four common causes of high anion gap metabolic acidosis?
Ketoacidosis, lactic acidosis, renal failure, and toxic ingestions
39. How is ketoacidosis typically treated in the setting of high anion gap acidosis?
By administering a slow infusion of insulin
40. What is the treatment for lactic acidosis with high anion gap?
Sodium bicarbonate infusion
41. What is the preferred treatment for high anion gap acidosis due to renal failure?
Dialysis
42. How are toxic ingestions managed in high anion gap acidosis?
Gastric lavage and activated charcoal
43. What is another name for normal anion gap metabolic acidosis?
Hyperchloremic acidosis
44. In normal anion gap acidosis, why is serum chloride elevated?
The kidneys reabsorb chloride in place of lost bicarbonate
45. What lab finding confirms hyperchloremic metabolic acidosis?
Low HCO₃⁻ with high chloride and a normal anion gap
46. What is the normal serum chloride level?
96 to 106 mEq/L
47. What are common causes of normal anion gap acidosis?
Severe diarrhea, renal bicarbonate loss, and impaired acid excretion
48. What metabolic process explains the increase in HCO₃⁻ when PaCO₂ acutely rises by 10 mmHg?
Hydrolysis effect
49. What does buffer base represent in acid-base physiology?
The total buffering capacity of the blood
50. Aside from the bicarbonate buffer system, what are other types of buffering systems in the blood?
Proteins, phosphates, and other closed buffer systems
51. What does bicarbonate buffer in the blood?
Hydrogen ions (H⁺)
52. What chemical equation represents the buffering of carbonic acid (H₂CO₃)?
CO₂ + H₂O → H₂CO₃ → H⁺ + HCO₃⁻
53. What is a major disadvantage of closed buffering systems?
Hydrogen ions have nowhere to go, potentially causing overload
54. How does buffer base respond to an increased fixed acid load?
It decreases, similar to the drop in bicarbonate
55. What happens to buffer base during metabolic alkalosis?
It increases due to an excess of base in the blood
56. What factor primarily affects the overall buffering capacity of blood?
Hemoglobin concentration
57. What is the approximate buffer base level in a person with normal hemoglobin levels?
48 mmol/L
58. What happens to buffer base when hemoglobin is decreased to half of normal?
It decreases to about 45 mmol/L
59. What effect does doubling the hemoglobin concentration have on buffer base?
It increases buffer base to around 50 mmol/L
60. What does base excess represent in acid-base analysis?
The difference between actual buffer base and normal expected buffer base
61. What does the value of base excess indicate clinically?
How much acid or base must be added to return the pH to 7.40 if PaCO₂ is 40 mmHg
62. If pH is elevated and PaCO₂ is 40 mmHg, what is the likely cause of alkalemia?
A metabolic process (non-respiratory) increasing base levels
63. A base excess of +8 mEq/L indicates what acid-base condition?
Metabolic alkalosis
64. If pH is decreased and PaCO₂ is 40 mmHg, what is the likely cause of acidemia?
A metabolic base deficit
65. A base deficit of −8 mEq/L indicates what condition?
Metabolic acidosis due to decreased base
66. How is a base deficit value typically expressed?
As a negative value, e.g., BE −8
67. What is the main drawback of interpreting base excess (BE) in isolation?
It can be misleading; for example, renal compensation for respiratory acidosis may appear as primary metabolic alkalosis if the full ABG and clinical context are not considered.
68. If PaCO₂ is 40 mmHg, how much does pH change for every 5 mmol/L change in base excess?
The pH changes by approximately 0.1 unit for every 5 mmol/L change in BE.
69. What is the base excess value typically associated with a pH of 7.30?
Approximately −5 mmol/L
70. What is the base excess value typically associated with a pH of 7.50?
Approximately +5 mmol/L
71. How does base excess generally correlate with bicarbonate (HCO₃⁻) levels?
Base excess moves in the same direction as HCO₃⁻; a decrease indicates metabolic acidosis, and an increase indicates metabolic alkalosis.
72. Why might base excess be falsely low during acute hypercapnia even though it remains within normal range?
Because BE calculations assume a PaCO₂ of 40 mmHg, and real physiological buffer shifts aren’t fully captured under lab conditions.
73. During acute increases in PaCO₂, how does base excess respond?
Base excess decreases by approximately 1 mmol/L for every 10 mmHg increase in PaCO₂.
74. How is base excess calculated by blood gas analyzers?
Using hemoglobin concentration, buffering capacity, intra- and extracellular buffering, bicarbonate level, CO₂ solubility, and the Siggaard-Andersen nomogram.
75. What does an arterial blood gas (ABG) test measure?
The partial pressures of oxygen (PaO₂), carbon dioxide (PaCO₂), blood pH, and bicarbonate (HCO₃⁻) in arterial blood.
76. Which four values are typically reported in an ABG?
PaO₂, PaCO₂, pH, and HCO₃⁻
77. What is acidosis?
A pathologic state characterized by an increase in hydrogen ions (H⁺) in arterial blood.
78. What causes the harmful effects in acidosis?
The presence of unbound hydrogen ions (H⁺) in the bloodstream.
79. At what pH value is blood considered to be acidotic?
When pH falls below 7.35
80. What are three acids involved in acid-base balance?
Hydrogen ions (H⁺), carbon dioxide (CO₂), and carbonic acid (H₂CO₃)
81. Through which organs are acids removed from the body?
The lungs eliminate volatile acids like CO₂, while the kidneys excrete fixed acids.
82. When are acids produced in the body?
During normal metabolic processes
83. What is alkalosis?
A pathologic state characterized by a decrease in hydrogen ion (H⁺) concentration in arterial blood.
84. At what pH value is blood considered alkalotic?
When pH rises above 7.45
85. What ion concentration decreases during alkalosis?
Hydrogen ions (H⁺)
86. What is the anion gap?
The difference between measured serum cations and anions, used to identify causes of metabolic acidosis.
87. What does a negative base excess value indicate?
A base deficit, meaning there is a deficient amount of bicarbonate in the blood.
88. What is an example of a base in the blood?
Bicarbonate (HCO₃⁻)
89. What is a buffer?
A substance that minimizes changes in pH by binding or releasing hydrogen ions (H⁺).
90. What do buffers bind or release to help maintain pH balance?
Hydrogen ions (H⁺)
91. What is the primary goal of buffer systems in the blood?
To maintain pH within the normal range
92. What are compensatory mechanisms in acid-base balance?
Physiological responses that prevent drastic pH changes and attempt to correct imbalances
93. What does a positive base excess value suggest in terms of acid-base balance?
It indicates a metabolic alkalosis or a gain in buffering base in the blood.
94. What clinical condition might be associated with a base excess of +10 mmol/L?
A metabolic alkalosis due to excessive bicarbonate retention or loss of acids (e.g., vomiting).
95. What base excess value would suggest a significant metabolic acidosis?
A base excess less than −10 mmol/L
96. How does base excess help differentiate between respiratory and metabolic causes of pH imbalance?
By isolating the non-respiratory component, since base excess is independent of PaCO₂.
97. Why is base excess considered a better indicator of metabolic status than HCO₃⁻ alone in critically ill patients?
Because it accounts for all buffer systems, not just bicarbonate.
98. What is the expected base excess in a patient with uncompensated respiratory acidosis?
Near normal or slightly decreased, depending on duration and compensation.
99. How does chronic respiratory alkalosis affect base excess?
It may cause a mild decrease in base excess due to renal compensation (loss of HCO₃⁻).
100. In what clinical scenarios is base excess particularly helpful?
In trauma, sepsis, and metabolic shock states to evaluate the severity of metabolic acidosis or alkalosis.
Final Thoughts
Base excess (BE) is an essential parameter in respiratory care, offering a window into the metabolic side of acid-base balance. It provides key insights that help respiratory therapists and other clinicians assess, diagnose, and manage patients with complex acid-base disturbances.
By recognizing whether an abnormality stems from a metabolic or respiratory origin and whether compensation is occurring, respiratory therapists can contribute meaningfully to decisions about ventilation strategies, fluid therapy, medication use, and overall patient stabilization.
Understanding BE and its clinical implications empowers respiratory professionals to deliver safer, more effective care—particularly in critical care, emergency medicine, and pulmonary rehabilitation settings.
Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
References
- Langer T, Brusatori S, Gattinoni L. Understanding base excess (BE): merits and pitfalls. Intensive Care Med. 2022.