A blood gas analyzer is an essential diagnostic tool in modern healthcare, particularly in respiratory care. It provides rapid and accurate measurements of critical blood parameters such as oxygen (O₂), carbon dioxide (CO₂), and pH.
These values give clinicians immediate insight into a patient’s respiratory and metabolic status, making the analyzer one of the most relied-upon devices in intensive care units, emergency departments, and respiratory therapy practice.
Take our free course to master the basics of ABG interpretation with clear explanations and helpful practice questions.
What Is a Blood Gas Analyzer?
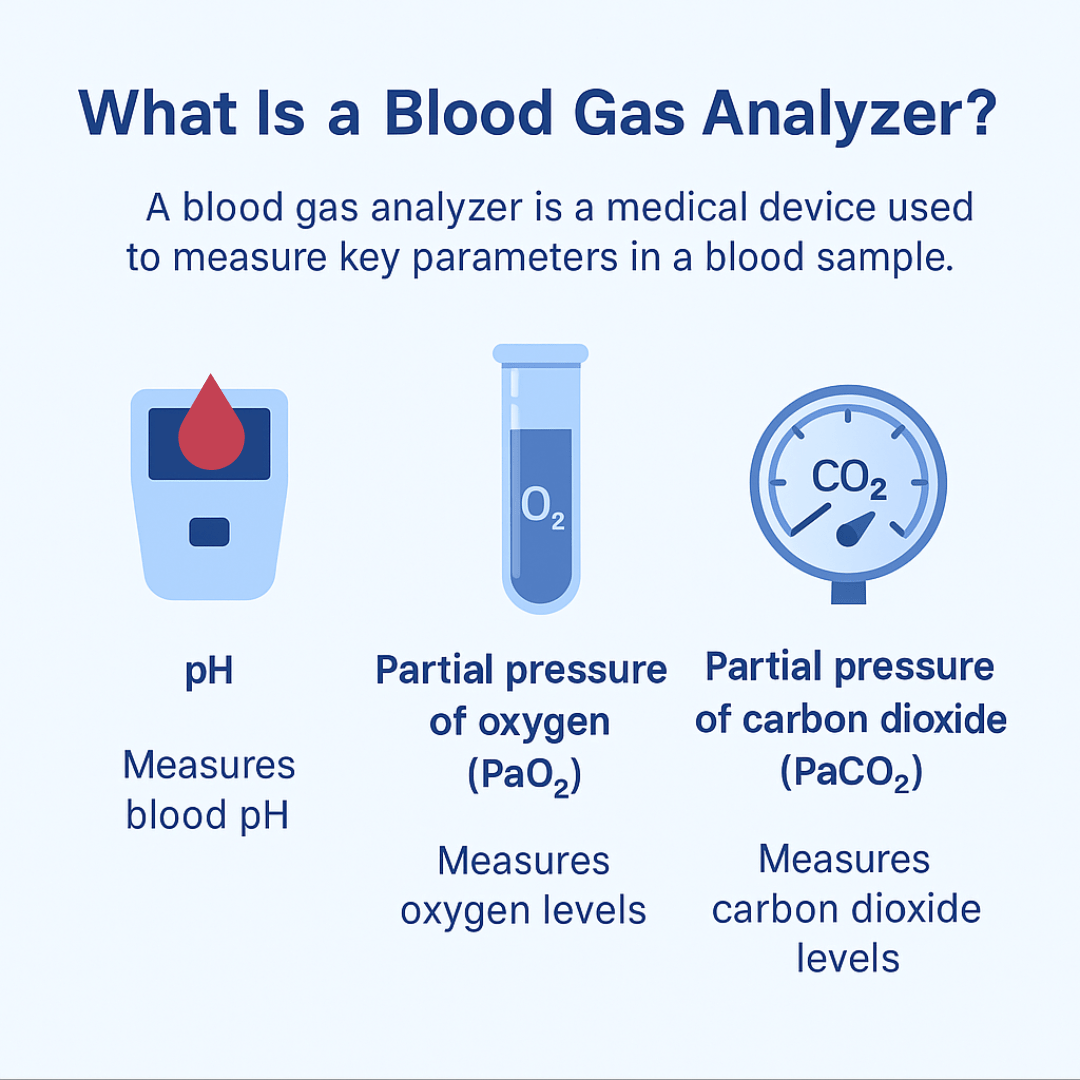
A blood gas analyzer is a medical device used to measure key parameters in a blood sample, typically arterial, to assess a patient’s respiratory and metabolic status. It provides rapid results for values such as pH, partial pressure of oxygen (PaO₂), partial pressure of carbon dioxide (PaCO₂), bicarbonate (HCO₃⁻), and oxygen saturation (SaO₂).
These measurements help determine how effectively the lungs are exchanging gases and how well the body is maintaining acid-base balance. Some advanced analyzers also measure electrolytes, lactate, and hemoglobin. The information gained is critical for guiding treatment decisions in respiratory therapy and critical care.
Blood Gas Parameters
A blood gas analyzer is a medical device that measures arterial, venous, or capillary blood samples to determine important physiological values. The most common parameters include:
- Partial pressure of oxygen (PaO₂) – reflects how well oxygen is moving from the lungs into the blood.
- Partial pressure of carbon dioxide (PaCO₂) – indicates how effectively CO₂ is being removed by the lungs.
- pH – reveals the acid-base balance of the blood.
- Bicarbonate (HCO₃⁻) – evaluates the metabolic component of acid-base balance.
- Oxygen saturation (SaO₂) – shows the percentage of hemoglobin bound with oxygen.
Note: Advanced analyzers may also measure electrolytes (sodium, potassium, chloride), lactate, hemoglobin levels, and other markers of tissue perfusion and oxygenation.
Why Is It Important in Respiratory Care?
For respiratory therapists, blood gas analysis is central to patient management. It provides a direct window into how the lungs and body are functioning in real time. The information guides decisions about oxygen therapy, ventilator settings, and overall treatment strategies. A few key examples include:
- Assessing oxygenation and ventilation: If PaO₂ is low, oxygen delivery may need to be increased. If PaCO₂ is elevated, it may suggest hypoventilation or inadequate ventilatory support.
- Monitoring mechanical ventilation: Blood gas results confirm whether a ventilator is set appropriately or needs adjustment.
- Evaluating acid-base status: Identifying respiratory acidosis, metabolic acidosis, alkalosis, or mixed disorders helps determine whether the problem is primarily respiratory, metabolic, or both.
- Emergency care: In acute situations such as asthma exacerbations, COPD flare-ups, or cardiac arrest, blood gas values provide immediate feedback about the patient’s condition and response to interventions.
Relevance to Respiratory Therapists
Respiratory therapists are often responsible for drawing arterial blood samples and operating the analyzer. Beyond just collecting data, they must interpret results and integrate them into patient care.
For example:
- A low PaO₂ with normal PaCO₂ may point to diffusion impairment or shunting.
- A high PaCO₂ with low pH suggests hypoventilation and respiratory acidosis.
- Abnormal HCO₃⁻ levels indicate metabolic contributions to acid-base imbalance.
Note: By understanding these results, respiratory therapists can collaborate with physicians to adjust oxygen therapy, modify ventilator settings, or recommend further testing.
Blood Gas Analyzer Electrodes
1. Clark Electrode – Oxygen Measurement
- Purpose: Measures the partial pressure of oxygen (PaO₂).
- How it works: Oxygen diffuses through a membrane and is reduced at a platinum cathode, creating an electrical current proportional to oxygen concentration.
- Clinical role: Provides direct insight into a patient’s oxygenation status and the effectiveness of oxygen therapy or mechanical ventilation.
2. Severinghaus Electrode – Carbon Dioxide Measurement
- Purpose: Measures the partial pressure of carbon dioxide (PaCO₂).
- How it works: It is essentially a modified pH electrode covered by a thin film of bicarbonate solution and a CO₂-permeable membrane. As CO₂ diffuses in, it alters the pH of the solution, and this pH change is measured to calculate PaCO₂.
- Clinical role: Essential for evaluating ventilation and ensuring patients are neither hypoventilating nor hyperventilating.
3. pH Electrode – Acid-Base Balance
- Purpose: Measures blood pH directly.
- How it works: A glass electrode sensitive to hydrogen ion concentration compares the sample against a reference electrode, producing a voltage that reflects blood pH.
- Clinical role: Critical for detecting acidosis, alkalosis, and understanding whether the disorder is primarily respiratory or metabolic.
Why They Matter Together
These three electrodes — Clark (O₂), Severinghaus (CO₂), and pH electrode — form the backbone of arterial blood gas (ABG) analysis. From their combined measurements, blood gas analyzers can also calculate secondary values such as bicarbonate (HCO₃⁻), base excess, and oxygen saturation (SaO₂).
This triad gives respiratory therapists and clinicians a comprehensive picture of gas exchange and acid-base balance, guiding life-saving interventions in critical care, emergency medicine, and respiratory therapy.
Blood Gas Analyzer Practice Questions
1. Which parameters are directly measured by a blood gas analyzer?
PaCO₂, PaO₂, and pH
2. What does PaCO₂ represent in a blood gas analysis?
The partial pressure of carbon dioxide in arterial blood
3. What does PaO₂ represent in a blood gas analysis?
The partial pressure of oxygen in arterial blood
4. What does pH represent in a blood gas analysis?
The hydrogen ion concentration, indicating acid-base balance
5. Which electrode in a blood gas analyzer measures PaCO₂?
Severinghaus electrode
6. Which electrode in a blood gas analyzer measures PaO₂?
Clark electrode
7. Which electrode in a blood gas analyzer measures pH?
Sanz electrode
8. How is calibration performed on a blood gas analyzer?
By using solutions or gases with known low and high values to ensure accuracy
9. What are two factors that can interfere with automatic calibration of a blood gas analyzer?
Low calibration gas levels and insufficient buffer solution
10. What are the three quality control levels used in blood gas analyzers?
Acidotic, Normal, and Alkalotic
11. How often should each level of quality control be performed on a blood gas analyzer?
At least once per day
12. How many data points are typically plotted before evaluating analyzer performance?
20 to 30 consecutive values
13. What is the name of the chart used to analyze quality control data from blood gas analyzers?
Levey-Jennings Chart
14. What is the purpose of the Levey-Jennings chart in blood gas analysis?
To detect whether the machine is functioning within control limits
15. What standard deviation range is typically used on Levey-Jennings charts to assess analyzer control?
±2 standard deviations
16. What does it mean if all values on a Levey-Jennings chart remain within 2 standard deviations?
The analyzer is considered “in control” and functioning properly.
17. What does it indicate if only one value falls outside the 2 SD range during quality control?
A random error has occurred; no action is necessary.
18. What does a trend pattern on a Levey-Jennings chart suggest, and what action should be taken?
Progressively increasing or decreasing values still within 2 SD; check analyzer for maintenance.
19. What does a shift pattern on a Levey-Jennings chart suggest, and what action should be taken?
A consistent deviation from the original mean but still within 2 SD; no immediate action required
20. What does an out-of-control pattern on a Levey-Jennings chart indicate, and what should be done?
Two or more values fall outside the 2 SD range; recalibrate or replace the electrode.
21. What quality control method uses unknown samples sent to multiple labs with the same analyzer model?
Proficiency testing
22. What quality control method compares patient results on two or more analyzers in the same lab?
Multiple machine analysis
23. What should be done if significant differences are found during multiple machine analysis?
Investigate and identify the cause of the discrepancy.
24. What device validates blood gas analyzers by equilibrating known gas mixtures with blood or buffer?
Gas Exchange Validation Device (Tonometry)
25. When using a gas exchange validation device, how are expected gas tensions (in torr) calculated?
From the fractional concentrations of FiO₂ and FiCO₂ in the precision gas mixture
26. Which quality control method is considered the gold standard for verifying the accuracy of the Clark (PaO₂) electrode?
Tonometry
27. What is the term for laboratory testing performed at the patient’s bedside?
Point-of-care testing
28. What is one major benefit of point-of-care testing?
It reduces the time from blood sample collection to test result reporting
29. Which portable device is commonly used to perform arterial blood gas analysis at the bedside?
i-STAT system
30. How quickly can point-of-care devices like the i-STAT provide ABG results?
Within approximately 90 seconds
31. Besides ABGs, what additional parameters can point-of-care devices measure?
Chemistry, hematology, electrolytes, glucose, BUN, and clotting factors
32. What procedure is used to obtain blood samples or provide continuous infusion access?
Venipuncture (Phlebotomy)
33. Which vein is most commonly accessed during venipuncture due to its size and location?
Antecubital vein (anterior to the elbow)
34. Are most clinical blood samples venous or arterial in origin?
Venous
35. What type of procedure allows for continuous administration of fluids, medications, or nutrition?
Intravenous (IV) infusion
36. Through which sites can intravenous infusion be performed?
Any central vein, such as the femoral, jugular, or subclavian
37. Which site is most commonly used for routine intravenous infusion?
Peripheral veins in the arm or hand
38. What three parameters are directly measured by standard blood gas analyzers?
pH, PaCO₂, and PaO₂
39. What additional feature may some blood gas analyzers include for oxygen saturation assessment?
Co-oximetry for direct measurement of HbO₂ saturation
40. What technical precautions must be followed when operating a blood gas analyzer?
Maintain 37°C temperature, ensure leak-free tubing, avoid air bubbles, and perform regular calibration.
41. What does two-point calibration provide in blood gas analysis?
Accuracy and linearity across a range of values
42. According to protocol, when is one-point calibration recommended for a blood gas analyzer?
Immediately before performing a sample analysis
43. What is the function of the Clark electrode in a blood gas analyzer?
It measures PaO₂ via current changes during an oxidation-reduction reaction
44. At what oxygen concentration is the Clark electrode typically calibrated?
12% O₂ (which equals about 86 torr at sea level)
45. What is the function of the Sanz electrode in a blood gas analyzer?
It measures pH based on hydrogen ion activity against a reference solution
46. At what value is the Sanz electrode typically calibrated?
7.38 pH with a standard buffer solution; slope set at 6.838
47. What is the function of the Severinghaus electrode in blood gas analysis?
It measures PaCO₂ by detecting hydrogen ion concentration in a buffered solution
48. What is the typical calibration point for the Severinghaus electrode?
5% CO₂, which is approximately 36 torr
49. How are bicarbonate and base excess values determined in ABG analysis?
They are calculated from measured pH and PaCO₂ values.
50. Why is it important to enter accurate hemoglobin levels in blood gas analyzer software?
To improve the precision of base excess and oxygen content calculations
51. What is a key limitation of mainstream capnography devices?
They tend to be heavy and produce heat, requiring patient protection.
52. What are two common problems associated with sidestream capnography devices?
Moisture buildup and sampling flow rate inconsistencies
53. How does a sampling flow rate that is too fast affect sidestream capnography readings?
It can cause fresh gas dilution and falsely low CO₂ readings.
54. How does a sampling flow rate that is too slow affect sidestream capnography readings?
It may distort the waveform and reduce reading accuracy.
55. What gases are typically used to calibrate a capnometer?
Room air and a precision gas containing 5.5% CO₂
56. What should capnography waveform interpretation include?
Normal patterns, obstructed ventilation, hypo/hyperventilation, mechanical dead space, and curare clefts
57. What principle do capnometers use to detect carbon dioxide?
Infrared absorption
58. What value should the PETCO₂ closely correlate with during capnography?
The mixed alveolar CO₂ value
59. What are alveoli with dead space ventilation most comparable to in terms of CO₂ levels?
Room air, since they receive little or no CO₂ due to a lack of perfusion
60. What is the normal gradient between PaCO₂ and PETCO₂?
2–3 torr
61. What does a widened PaCO₂ – PETCO₂ gradient typically indicate?
Increased dead space ventilation (e.g., in pulmonary embolism or COPD)
62. At what temperature is blood gas analysis standardized and performed?
37°C
63. How does oxygen tension (PaO₂) change with temperature during blood gas analysis?
It varies directly by approximately 6% per degree Celsius.
64. How does carbon dioxide tension (PaCO₂) change with temperature differences?
It varies directly by approximately 4.5% per degree Celsius.
65. How does blood pH change with differences in patient temperature?
It varies inversely by approximately 0.014 units per degree Celsius.
66. What is used to verify the accuracy of a blood gas analyzer during quality control?
Commercial control solutions or tonometered blood with known values
67. What is the expected result of a quality control sample when run on a blood gas analyzer?
The value should fall within ±1 standard deviation of the mean.
68. When is a single QC result considered acceptable even if slightly off?
If it falls within ±2 standard deviations
69. What level of deviation on a QC test indicates an immediate warning?
Greater than +2 standard deviations
70. What QC result indicates the analyzer is out of control?
A result greater than +3 SD, or repeated patterns such as 2 consecutive +2 SD values
71. What QC pattern suggests an analyzer is out of control even if values are close to the mean?
10 consecutive values all above or below the mean, even if within 1 SD
72. What should be done when an electrode on a blood gas analyzer fails to meet QC standards?
Troubleshoot the specific electrode that is out of compliance.
73. What type of chart is used to track quality control results for blood gas analyzers?
Levey-Jennings chart
74. What is the purpose of proficiency testing in laboratory quality assurance?
To evaluate analyzer accuracy using unknown samples submitted to an external agency
75. What does a CO-oximeter use to analyze hemoglobin variants?
Spectrophotometry at multiple wavelengths after hemolyzing the blood
76. What scientific law is applied in spectrophotometry for CO-oximetry?
Beer’s Law, which states that absorbance is directly proportional to concentration
77. What four hemoglobin species are measured by a hemoximeter?
Oxyhemoglobin, reduced hemoglobin, carboxyhemoglobin, and methemoglobin
78. In carbon monoxide poisoning, how are ABG results affected without co-oximetry?
PaO₂ will be accurate, but oxygen saturation will be falsely elevated.
79. Why is co-oximetry necessary in cases of suspected CO poisoning?
To determine the true oxygen content and differentiate hemoglobin species
80. What does transcutaneous monitoring measure using skin-attached electrodes?
PaO₂ and PaCO₂ using technology similar to that in ABG analyzers
81. How does heating the skin to 44°C help in transcutaneous monitoring?
It increases capillary blood flow and skin permeability to gas diffusion.
82. How often should the site of a transcutaneous electrode be changed?
Every 4 hours
83. What can cause falsely low transcutaneous oxygen readings?
Decreased perfusion at the electrode site
84. What is the primary advantage of using transcutaneous CO₂ monitoring in all age groups?
It closely correlates with arterial CO₂, even in adults.
85. What electrode directly measures PaCO₂ in a blood gas analyzer?
Severinghaus electrode
86. What electrode directly measures PaO₂ in a blood gas analyzer?
Clark electrode
87. What electrode directly measures pH in a blood gas analyzer?
Sanz electrode
88. Which ABG parameters are calculated rather than directly measured?
SaO₂, HCO₃⁻, and Base Excess (BE)
89. How is calibration performed on an oxygen analyzer?
By exposing it to gases with known oxygen concentrations (low and high)
90. TRUE or FALSE: An oxygen analyzer must accurately read a known gas sample during calibration.
TRUE
91. What is the standard low calibration solution value for pH?
6.840
92. What must be done if the low calibration value is not met during analyzer setup?
Adjust the machine to match the known low standard.
93. After adjusting for the low standard in calibration, what is the next step?
Repeat the procedure using the high calibration solution.
94. How often is an automatic one-point calibration performed on a blood gas analyzer?
Every 30 minutes or before sample analysis
95. What additional calibration process occurs every 8 hours on a blood gas analyzer?
A two-point calibration follows the routine one-point calibration.
96. During one-point calibration, what pH value must the pH electrode achieve?
A value of 7.38 or within ±1 standard deviation of the mean, per manufacturer guidelines
97. What is the expected pH value during two-point calibration for the pH electrode?
A value of 6.84 or within ±1 standard deviation of the mean
98. What do the one-point and two-point calibrations establish for a blood gas analyzer?
They provide high and low reference values to ensure optimal electrode performance.
99. What are the substances used in internal calibration of a blood gas analyzer called?
Control media or quality control (QC) samples
100. How many quality control levels are typically used to verify the accuracy of a blood gas analyzer?
Three levels of QC to assess performance across a range of values
Final Thoughts
The blood gas analyzer is more than just a laboratory device—it is a cornerstone of respiratory care. Its ability to provide rapid, accurate, and actionable data makes it indispensable in managing critically ill patients and guiding life-saving interventions.
For respiratory therapists, mastering the use and interpretation of blood gas results is a fundamental skill that ensures better patient outcomes and enhances the quality of care.
Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
References
- Hassan W, Martinez S. Arterial Blood Gas Sampling [ABG Machine Use] [Updated 2024 May 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025.


