Bronchoalveolar lavage (BAL) is a valuable diagnostic and therapeutic procedure in respiratory care that involves washing out the lower airways with a sterile saline solution during bronchoscopy. By collecting fluid samples from the alveoli and bronchioles, clinicians gain critical insights into lung infections, inflammatory conditions, and certain malignancies.
This minimally invasive technique not only helps in identifying pathogens and cellular abnormalities but also plays a key role in guiding targeted treatment strategies.
For respiratory therapists and other healthcare providers, understanding BAL is essential, as it enhances patient assessment, supports accurate diagnosis, and contributes to the effective management of complex pulmonary diseases.
What Is Bronchoalveolar Lavage?
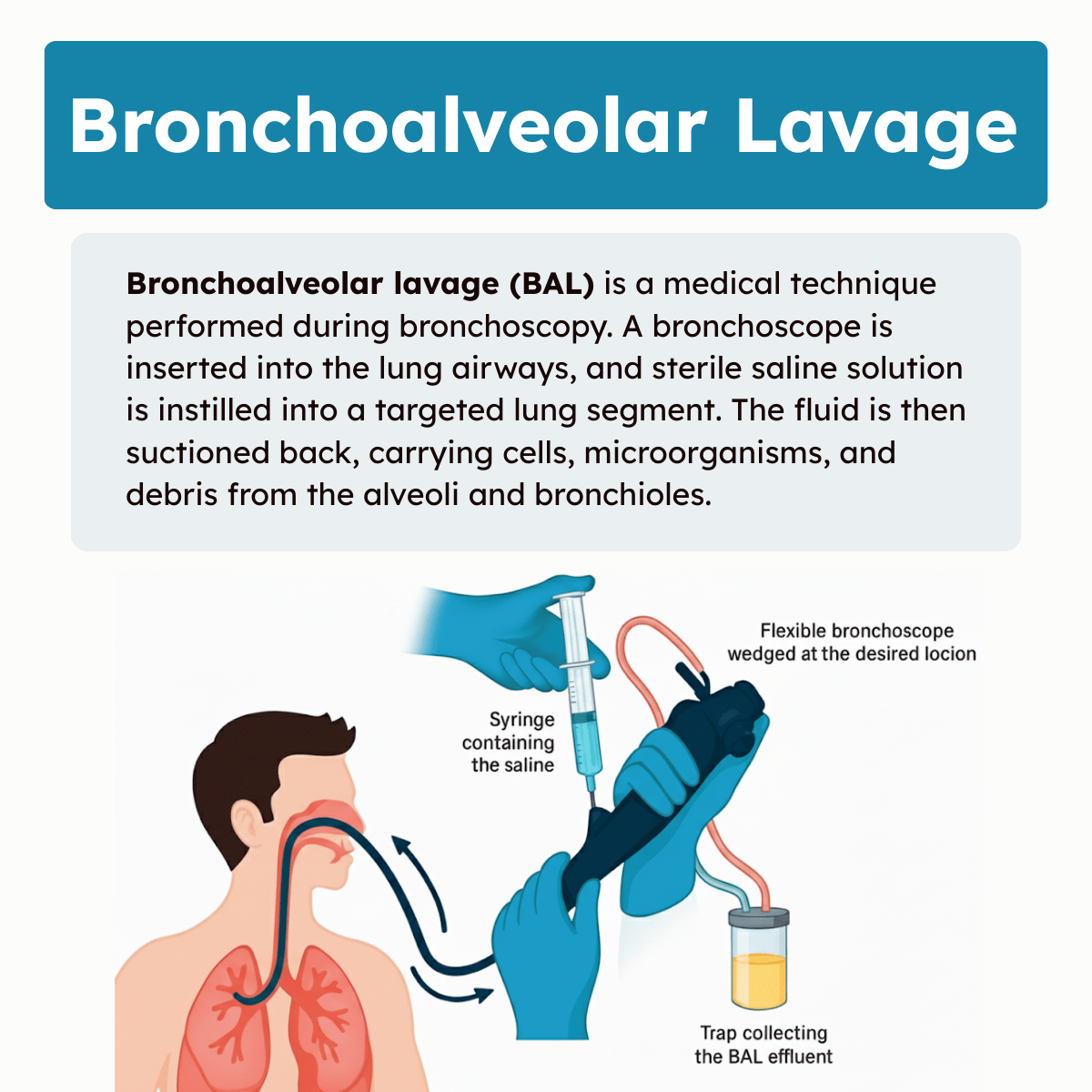
Bronchoalveolar lavage (BAL) is a medical technique performed during bronchoscopy. A bronchoscope is inserted into the lung airways, and sterile saline solution is instilled into a targeted lung segment. The fluid is then suctioned back, carrying cells, microorganisms, and debris from the alveoli and bronchioles.
The recovered fluid reflects the fluid environment of the lower respiratory tract. It is primarily used to analyze cellular content, infectious agents, and inflammatory markers. BAL differentiates lung conditions such as infections, malignancies, or interstitial lung diseases.
Purpose and Indications
BAL is used to diagnose infections, especially in immunocompromised patients. It identifies bacteria, viruses, fungi, and Pneumocystis jirovecii. It also helps detect malignancies and inflammatory diseases, such as sarcoidosis or pulmonary alveolar proteinosis.
Doctors use BAL when non-invasive tests, such as sputum analysis, are insufficient. It assists in evaluating unexplained lung infiltrates on imaging. The procedure can also monitor treatment effectiveness and disease progression in chronic lung diseases.
Bronchoalveolar Lavage Procedure
Bronchoalveolar lavage involves careful preparation, a precise technique, and specific methods for collecting samples. These elements ensure the procedure is effective and yields useful diagnostic information while minimizing patient risk.
Preparation and Patient Selection
The patient must undergo a thorough clinical evaluation before the procedure. This includes reviewing medical history, current medications, and coagulation status. Contraindications such as severe hypoxia, unstable cardiovascular status, or bleeding disorders must be ruled out. Patients are usually required to fast for at least 4-6 hours prior.
Local anesthesia and sedation are typically administered to reduce discomfort and coughing during the procedure. Oxygen supplementation may be provided as needed. Informed consent is obtained after explaining potential risks such as fever, bleeding, or transient hypoxia. The procedure is most often performed in an endoscopy suite with monitoring equipment.
Step-By-Step Technique
A flexible bronchoscope is inserted through the mouth or nose into the bronchial tree under direct visualization. The operator advances to the targeted lung segment, often identified by radiologic findings. Once positioned, sterile saline is instilled in aliquots (typically 20-60 mL each), with a total volume generally ranging from 100 to 300 mL. Each aliquot is gently suctioned back into sterile containers for analysis.
Throughout the lavage, lung compliance and patient vital signs are closely monitored. Care is taken to avoid excessive suction pressure to prevent airway trauma. After completion, the bronchoscope is carefully withdrawn, and the patient is observed until stable. The entire procedure often lasts 30-60 minutes.
Sample Collection Methods
Recovered lavage fluid is immediately placed in sterile containers and sent for multiple analyses. These may include microbiology cultures, cytology, and biochemical testing. Samples are often divided: one for bacterial, fungal, and viral cultures; another for cell differential counts and cytopathology. Additional aliquots may be used for molecular diagnostics.
Proper labeling and swift transport to the laboratory are essential to preserve sample integrity. Temperature control, such as refrigeration, can be necessary depending on downstream tests. The quality of specimen collection influences diagnostic yield and impacts patient management decisions.
Interpretation of Bronchoalveolar Lavage Results
The results of bronchoalveolar lavage (BAL) provide quantitative and qualitative data on cell populations, microorganisms, and cytology within the lower respiratory tract. Accurate interpretation involves understanding variations from normal ranges, identifying pathological markers, analyzing microbial presence, and assessing cellular abnormalities.
Normal Cell Counts
Normal BAL fluid typically contains macrophages as the predominant cell type, accounting for approximately 85–95% of the total cells. Lymphocytes generally constitute 5–15%, while neutrophils and eosinophils are typically low, each accounting for less than 3%.
Variations within these ranges depend on the lung region sampled and patient factors such as smoking status. Persistent elevations in inflammatory cells outside these normal limits may imply lung pathology.
Note: Interpretation must consider the balance among these populations rather than isolated values.
Pathological Findings
Increases in neutrophils suggest acute inflammation, as seen in bacterial infections or acute respiratory distress syndrome (ARDS). Elevated eosinophils are indicators of eosinophilic pneumonia or hypersensitivity reactions.
Lymphocytosis in BAL fluid often points to chronic inflammatory or autoimmune disorders like sarcoidosis or hypersensitivity pneumonitis. The presence of atypical cells or excessive cellular debris may also indicate malignancy or alveolar damage.
Microbiological Analysis
BAL fluid is cultured to detect bacterial, viral, fungal, and mycobacterial organisms. It allows for direct identification of pathogens causing pneumonia or opportunistic infections in immunocompromised patients.
Quantitative cultures help distinguish colonization from infection. Polymerase chain reaction (PCR) and antigen tests on BAL specimens provide rapid pathogen detection, improving diagnostic accuracy for difficult-to-culture organisms.
Cytological Assessment
Cytology of BAL fluid examines cell morphology for malignancy, infection, and immune response. Malignant cells may appear with irregular nuclei, hyperchromasia, and abnormal mitoses.
Infectious agents like Pneumocystis jirovecii or viral inclusions can be identified by specific staining techniques. Cytology also aids in detecting alveolar hemorrhage, granulomatous inflammation, or drug-induced lung injury through characteristic cellular changes.
Clinical Applications of Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) allows targeted sampling from the lower respiratory tract, providing critical information about lung pathology. It is widely used for diagnosing infections, evaluating interstitial lung diseases, and investigating malignancies.
Infectious Disease Diagnosis
BAL is essential for identifying pathogens in patients with pneumonia, especially when noninvasive methods fail. It enables direct sampling of alveolar secretions to detect bacteria, viruses, fungi, and parasites.
In immunocompromised patients, BAL improves the detection of opportunistic infections such as Pneumocystis jirovecii and cytomegalovirus. Microbiological cultures, PCR, and staining techniques applied to BAL fluid increase diagnostic accuracy.
BAL also guides antimicrobial therapy by identifying specific organisms and resistance patterns. This method reduces diagnostic uncertainty in ventilator-associated pneumonia and other complex pulmonary infections.
Interstitial Lung Disease Evaluation
BAL helps differentiate types of interstitial lung diseases (ILD) by analyzing cellular profiles within the lung environment. It measures proportions of lymphocytes, neutrophils, eosinophils, and macrophages, reflecting ongoing inflammation or fibrosis.
Specific patterns, such as lymphocytosis in hypersensitivity pneumonitis or neutrophilia in idiopathic pulmonary fibrosis, assist in diagnosis. BAL complements imaging and tissue biopsy for a comprehensive ILD assessment.
Note: Cellular analysis from BAL can also monitor disease progression or response to treatment, aiding in patient management over time.
Oncological Investigations
BAL can identify malignant cells in suspected lung cancer cases, particularly when tumors are centrally located or in non-resectable areas. Cytological examination of lavage fluid aids in confirming malignancy without more invasive procedures.
It also helps detect secondary pulmonary involvement in systemic cancers by revealing metastatic cells. BAL supports appropriate staging and treatment decisions. When combined with biomarkers and molecular testing, BAL contributes valuable information for targeted therapies in lung cancer patients.
Complications and Safety Considerations
Bronchoalveolar lavage (BAL) carries certain risks that vary by patient health and procedural factors. Identifying contraindications and knowing how to manage complications promptly ensures patient safety during and after the procedure.
Potential Risks
BAL can cause hypoxemia due to the temporary disruption of gas exchange in the lavaged lung segment. Bronchospasm is a common reaction, particularly in patients with reactive airways, and may require the use of bronchodilators.
Bleeding can occur from mucosal trauma or underlying coagulopathy. The risk of infection is low but present if sterile technique is breached. Rarely, pneumothorax may result from airway trauma or alveolar rupture during the lavage process.
Note: Monitoring oxygen saturation and clinical signs throughout the procedure helps detect complications early. The risk of serious adverse events is low in carefully selected patients.
Contraindications
Severe hypoxemia with PaO2 less than 60 mmHg on supplemental oxygen is a relative contraindication due to increased procedural risk. Unstable cardiovascular status or recent myocardial infarction also contraindicates BAL.
Patients with uncontrolled coagulopathy or severe thrombocytopenia (platelets <50,000/µL) should avoid BAL to reduce bleeding risk. Suspicion of bronchopleural fistula is another contraindication because lavage may worsen air leaks.
Severe bronchospasm or wheezing not responsive to bronchodilators requires deferral until better control is achieved. Inability to cooperate or protect the airway poses significant procedural challenges.
Management of Adverse Events
For hypoxemia, supplemental oxygen or temporary procedure interruption can stabilize the patient. Bronchospasm responds to inhaled beta-agonists and, if severe, corticosteroids. Bleeding is managed with local saline lavage and, in some cases, topical vasoconstrictive agents. If bleeding is significant, the procedure should be stopped and hemodynamic monitoring intensified.
Pneumothorax requires prompt chest imaging and, if large or symptomatic, insertion of a chest tube. Maintaining close observation post-procedure for delayed complications is critical for timely intervention.
Bronchoalveolar Lavage Practice Questions
1. What is bronchoalveolar lavage (BAL)?
A procedure that collects lower respiratory tract specimens using saline instillation and aspiration during bronchoscopy.
2. Why is bronchoalveolar lavage often performed?
To evaluate lung and airway diseases or investigate infections in immunocompromised patients.
3. How is a BAL sample obtained?
Sterile saline is injected into the alveolar space through a bronchoscope, mixed, and then aspirated for analysis.
4. Which lung disorder is characterized by alveoli filled with floccular, proteinaceous material and can be identified by BAL?
Pulmonary alveolar proteinosis (PAP).
5. What is the dominant cell type found in a normal BAL sample?
Macrophages, typically comprising 55–80% of cells.
6. Which patients are most likely to undergo BAL to diagnose opportunistic infections?
Immunocompromised patients, such as those with HIV or post-transplant.
7. What are common indications for bronchoalveolar lavage?
Diffuse lung infiltrates, ventilator-associated pneumonia (VAP), suspected alveolar hemorrhage, or suspected malignancy.
8. Which type of bronchoscope is most often used to perform BAL?
A flexible bronchoscope.
9. What is the purpose of a mini-BAL procedure?
To obtain distal lung specimens for diagnosing ventilator-associated pneumonia (VAP).
10. What specialized catheter is used in a mini-BAL procedure to prevent contamination?
A protected specimen catheter, such as a Combicath.
11. How much fluid is typically instilled during a BAL for cellular analysis and culture?
100–300 mL in 20–50 mL aliquots of sterile saline.
12. Why is the first aliquot of BAL fluid often discarded?
Because it contains mostly bronchial secretions and provides less diagnostic yield.
13. What is the ideal fluid return percentage during BAL?
Approximately 50–70% of the instilled saline.
14. How should BAL specimens be transported to the laboratory if delivered within 30 minutes?
At room temperature.
15. How should BAL specimens be stored if analysis will be delayed longer than 30 minutes?
On ice, but testing should not be delayed more than 24 hours.
16. What should be checked during physical examination of BAL fluid?
Evidence of hemorrhage, clots, or abnormal coloration.
17. What does a milky or beige BAL sample suggest?
Excess phospholipid proteins, as seen in pulmonary alveolar proteinosis.
18. How soon should cell counts from BAL be performed?
Within 1 hour using hemocytometry or automation, or up to 3 hours if placed in media.
19. Which white blood cell is normally the most abundant in BAL samples?
Macrophages
20. What percentage of lymphocytes is typically seen in BAL fluid from healthy individuals?
1–15%
21. Which infections are commonly diagnosed with BAL in immunocompromised patients?
Pneumocystis jirovecii and Cryptococcus neoformans.
22. What does the presence of hemosiderin-laden macrophages in BAL indicate?
Alveolar hemorrhage
23. Which additional findings in BAL fluid may suggest occupational exposure?
Dust particles ingested by macrophages.
24. Why is differential cell count performed on BAL samples?
To identify abnormal distributions of macrophages, lymphocytes, neutrophils, eosinophils, or malignant cells.
25. How many cells are typically counted in a BAL differential analysis?
300–1000 white blood cells.
26. What does an increased eosinophil count in BAL fluid suggest?
Possible allergic or eosinophilic lung disease.
27. What type of epithelial cells may appear in BAL samples?
Bronchial and squamous epithelial cells.
28. Which lung segment is commonly chosen for BAL collection?
The right middle lobe or lingula.
29. What common respiratory pathogens can BAL detect?
Fungal, viral, and bacterial organisms.
30. What should be done if BAL specimens cannot be analyzed immediately?
They should be centrifuged, resuspended in nutrient-supplemented media, and stored appropriately for testing.
31. What color might BAL fluid from a heavy smoker appear?
Gray
32. A milky or beige-colored BAL fluid is most often associated with which condition?
Pulmonary alveolar proteinosis.
33. What is the most prominent cell type normally seen in BAL fluid?
Macrophages
34. An increase in neutrophils in BAL fluid suggests which condition?
Aspiration pneumonia or acute bacterial infection.
35. The presence of red blood cells in BAL fluid indicates what?
Alveolar hemorrhage
36. Which disease cannot be diagnosed using BAL fluid analysis?
Meningitis
37. What procedure is used to perform a bronchoalveolar lavage?
Bronchoscopy
38. In BAL, what is done to the targeted lung segment?
It is rinsed with sterile saline.
39. An orange-red BAL fluid sample most likely indicates what?
Older alveolar hemorrhage.
40. Within what time frame should BAL cell counts ideally be performed?
Within 1 hour of collection.
41. An elevated CD4/CD8 lymphocyte ratio in BAL fluid is suggestive of what disease?
Sarcoidosis
42. Which laboratory method is used for immunological study of BAL cells?
Flow cytometry
43. What is the opportunistic fungal pathogen often recovered in BAL samples from AIDS patients?
Cryptococcus neoformans
44. Which stain is commonly used in BAL cytology to detect lipid-laden macrophages?
Sudan III stain
45. In BAL differential cell counts, which cell type normally makes up 55–80%?
Macrophages
46. A BAL fluid showing increased neutrophils and columnar epithelial cells from a construction worker likely contains which other abundant cell type?
Macrophages
47. Inclusions resembling dust-like particles in BAL macrophages are most associated with what?
Occupational exposure, such as asbestos or dust inhalation.
48. In a patient with suspected occupational lung disease, what BAL finding would support asbestos toxicity?
Gray fluid with increased neutrophils and dust-laden macrophages.
49. In BAL evaluation, what is considered the normal saline instillation volume?
100–300 mL
50. How are BAL volumes typically administered during the procedure?
In 20–50 mL aliquots of sterile saline.
51. True or False: The first aliquot of BAL fluid is discarded because it contains mostly bronchial secretions.
True
52. Besides microbiological studies, what additional diagnostic information can BAL provide?
Cellular and immunologic information from the lower respiratory tract.
53. Which imaging technique is often used with BAL to determine whether a biopsy is needed?
High-resolution computed tomography (HRCT).
54. Which six conditions can BAL help evaluate?
Interstitial lung disease, airway disease, alveolar hemorrhage, pulmonary alveolar proteinosis, Langerhans cell histiocytosis, and dust or asbestos exposure.
55. What is one advantage of BAL compared to surgical lung biopsy?
It is less invasive and provides cellular and microbiological data.
56. Which BAL finding supports the diagnosis of pulmonary alveolar proteinosis?
Milky, protein-rich fluid with increased phospholipid material.
57. Why is it important to record the segment of the lung used in BAL?
To correlate findings with radiographic abnormalities and ensure accurate diagnosis.
58. How are BAL specimens typically transported to the laboratory?
At room temperature if processed quickly, or on ice if delayed more than 30 minutes.
59. Why should BAL specimens not be tested after 24 hours?
Because cellular integrity and microbial viability decline, reducing diagnostic accuracy.
60. What is the primary role of a fiberoptic bronchoscope in BAL?
To guide saline instillation and recovery from the targeted lung segment.
61. What is the desired minimum volume of fluid recovered during a BAL?
At least 5 mL, with an ideal range of 10–20 mL.
62. How should BAL specimens be stored and transported if delivered immediately?
At room temperature.
63. What should be done if BAL delivery to the lab takes longer than 30 minutes?
Store the sample at 4°C (on ice).
64. When should cell counts from BAL specimens be performed?
Within 1 hour of collection.
65. How long can BAL cells remain stable if stored in nutrient-supplemented media?
Up to 3 hours.
66. What types of diagnostic tests can be performed on BAL fluid?
Cell counts, differential counts, microbiology studies, and cytopathology.
67. What are the possible colors of BAL fluid?
Clear, milky white, light brown beige, gray beige, and red.
68. What does a gray-beige BAL fluid sample typically indicate?
The patient is a smoker.
69. What does a red BAL fluid sample suggest?
Acute diffuse alveolar hemorrhage.
70. What condition is suggested by orange-red BAL fluid?
Older hemorrhagic syndrome.
71. A milky or light brown BAL sample is associated with which condition?
Pulmonary alveolar proteinosis.
72. What are the four possible clarity descriptions for BAL fluid?
Clear, hazy, cloudy, or turbid.
73. Which cells are commonly counted in BAL analysis?
White blood cells and red blood cells.
74. What dye is used to assess cell viability in BAL samples?
Trypan blue
75. How are WBCs diluted for BAL counting?
Using BMP LeukoChek, which provides a 1:100 dilution with ammonium oxalate.
76. What is the purpose of ammonium oxalate in BAL WBC dilution?
To lyse red blood cells before counting.
77. How long should diluted BAL cells be allowed to settle before counting?
Five minutes
78. When counting WBCs from BAL fluid on a hemocytometer, how many squares should be counted?
All 18 squares on both sides.
79. What should be done after counting WBCs on both sides of a hemocytometer?
Calculate the average of the two counts.
80. How are RBCs diluted for BAL analysis?
With isotonic saline.
81. After diluting RBCs for BAL, what should be done before counting?
Plate the sample on a hemocytometer, allow it to settle for 5 minutes, and then count both sides.
82. What is the maximum acceptable difference between WBC counts from two hemocytometer squares?
No more than 15 cells.
83. What is the maximum acceptable difference between RBC counts from two hemocytometer squares?
No more than 30 cells.
84. True or False: BAL cell counts from each side of the hemocytometer must be within 10% of each other.
True
85. Which leukocytes are commonly noted in BAL samples?
Macrophages, CD4+ lymphocytes, CD8+ lymphocytes, neutrophils, eosinophils, ciliated columnar epithelial cells, and squamous epithelial cells.
86. What is the most prominent cell type in BAL samples?
Macrophages
87. What pigments may macrophages in BAL contain?
Hemosiderin, gold, brown, or black particles, often with a foamy appearance.
88. Why are increased macrophages often seen in BAL fluid?
Because of smoking-related exposure.
89. What is the normal percentage of lymphocytes in BAL fluid?
1–15%
90. An increase in BAL lymphocytes may be seen in which conditions?
Interstitial lung disease, drug reactions, pulmonary lymphoma, and nonbacterial infections.
91. A lymphocyte differential count greater than 25% in BAL fluid suggests what condition?
Granulomatous lung disease
92. A lymphocyte differential count greater than 50% in BAL fluid is most consistent with which disorders?
Nonspecific interstitial pneumonia or hypersensitivity pneumonitis.
93. What does an increased percentage of neutrophils in BAL fluid suggest?
Smoking, bronchopneumonia, toxin exposure, or alveolar damage.
94. Neutrophil counts greater than 50% in BAL fluid are associated with what conditions?
Acute lung injury, aspiration pneumonia, or suppurative infection.
95. Which patient history detail would support BAL testing for suspected asbestos exposure?
Occupational work in construction, demolition, or renovation of old buildings.
96. Why is BAL especially valuable in immunocompromised patients?
It helps identify opportunistic infections such as Pneumocystis jirovecii or fungal pathogens.
97. What finding in BAL fluid strongly suggests alveolar hemorrhage?
The presence of red blood cells and hemosiderin-laden macrophages.
98. Why might epithelial cells appear in BAL fluid?
Due to contamination from the upper airways or injury to bronchial tissue.
99. Which BAL finding is most consistent with hypersensitivity pneumonitis?
Marked lymphocytosis with a low CD4/CD8 ratio.
100. What is the clinical significance of dust-laden macrophages observed in BAL?
They indicate occupational lung disease, such as pneumoconiosis or asbestosis.
Final Thoughts
Bronchoalveolar lavage is an important procedure that helps clinicians gather direct information from the lower airways. By analyzing the fluid obtained, healthcare providers can identify infections, inflammatory conditions, or other abnormalities that may not be clear through imaging alone.
For respiratory therapists, having a working knowledge of BAL is useful for supporting patient care, assisting with the procedure, and understanding its role in the broader management of lung disease.
Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
References
- Patel PH, Antoine MH, Sankari A, et al. Bronchoalveolar Lavage. [Updated 2024 Feb 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025.

