Dead space ventilation is a fundamental concept in respiratory physiology that plays a critical role in understanding how effectively the lungs exchange gases. While breathing may appear straightforward, not all inhaled air participates in oxygen and carbon dioxide exchange. A portion of each breath is essentially “wasted” in terms of gas exchange, and this is known as dead space.
For respiratory therapists, recognizing, measuring, and managing dead space ventilation is essential for patient assessment, ventilator management, and optimizing overall respiratory care in both acute and chronic settings.
What Is Dead Space Ventilation?
Dead space ventilation refers to the portion of each breath that does not participate in gas exchange. This occurs when inhaled air remains in the conducting airways, such as the trachea and bronchi, or reaches alveoli that are ventilated but not adequately perfused with blood. As a result, this air does not contribute to oxygen uptake or carbon dioxide removal.
Dead space is a normal part of breathing and typically accounts for about one-third of each tidal volume in healthy individuals. However, when dead space increases due to conditions like pulmonary embolism, low cardiac output, or lung disease, ventilation becomes less efficient. This forces the body to increase breathing effort to maintain normal blood gas levels, which can contribute to respiratory distress and impaired gas exchange.
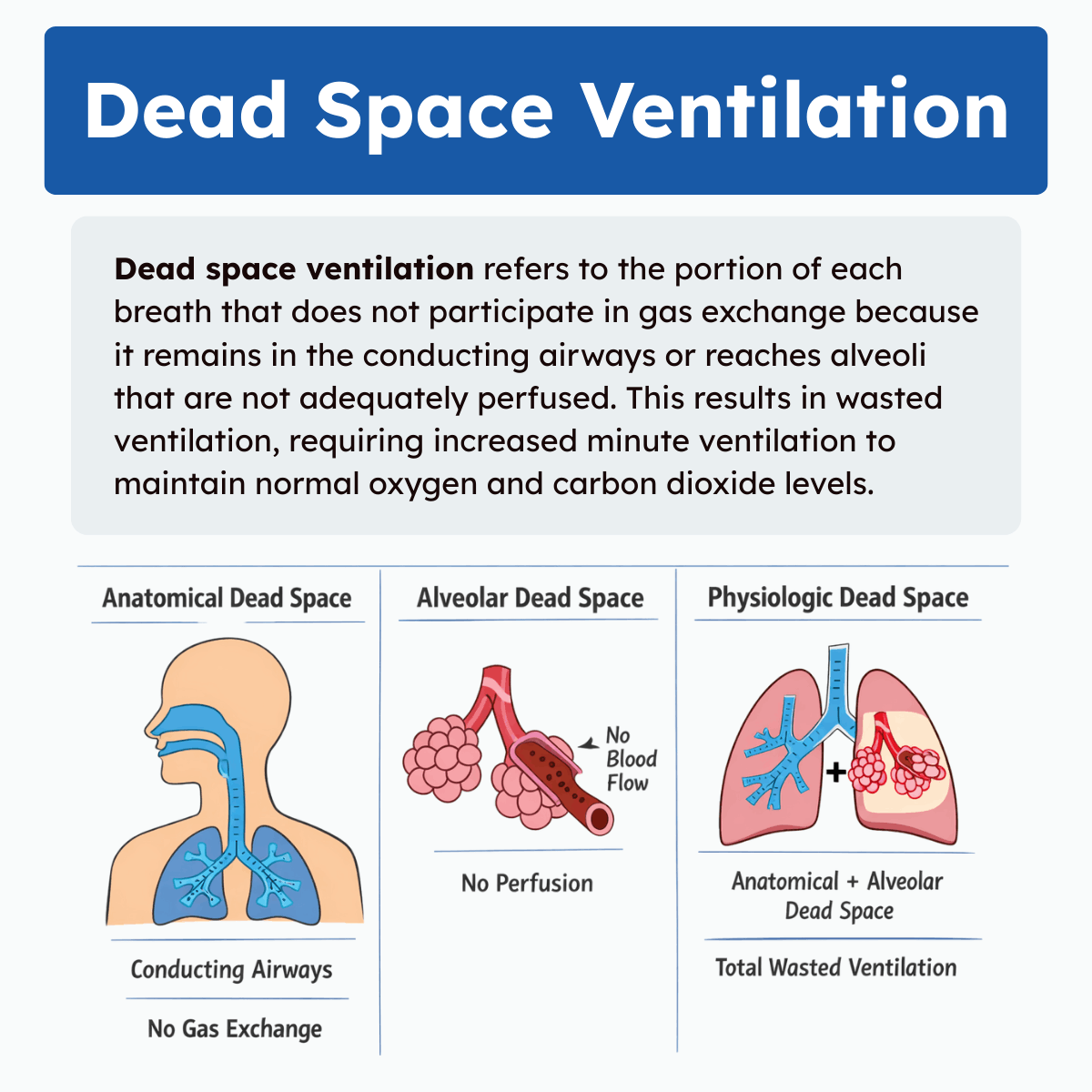
Types of Dead Space Ventilation
Dead space is commonly divided into three categories: anatomical dead space, alveolar dead space, and physiologic dead space. Each represents a different mechanism by which ventilation fails to contribute to gas exchange.
1. Anatomical Dead Space
Anatomical dead space is the volume of air that occupies the conducting airways of the respiratory system. These structures include the nose, pharynx, larynx, trachea, bronchi, and larger bronchioles. Although air flows through these passages during inhalation and exhalation, no gas exchange occurs because they lack alveoli.
When a person inhales, the first portion of air fills the conducting airways before fresh air reaches the alveoli. During exhalation, this same air is expelled first, still containing relatively high oxygen and low carbon dioxide levels compared to alveolar gas.
A commonly used estimate for anatomical dead space is approximately 1 mL per pound of ideal body weight. For example, an individual with an ideal body weight of 162 pounds would have an anatomical dead space of about 162 mL. This value remains relatively constant unless the airway anatomy is altered, such as with endotracheal intubation, tracheostomy, or artificial airway devices.
2. Alveolar Dead Space
Alveolar dead space occurs when air reaches the alveoli but does not participate in gas exchange due to inadequate or absent pulmonary perfusion. In this situation, ventilation is present, but blood flow is insufficient to allow oxygen uptake or carbon dioxide removal.
Under normal conditions, alveolar dead space is minimal. However, it can increase significantly in disease states that reduce pulmonary blood flow or disrupt normal ventilation-perfusion relationships.
Common causes of increased alveolar dead space include:
- Pulmonary embolism
- Low cardiac output states
- Shock or significant blood loss
- Congestive heart failure
- Severe hypotension
Note: When alveoli are ventilated but not perfused, they effectively behave like anatomical dead space, contributing to wasted ventilation and impaired gas exchange.
3. Physiologic Dead Space
Physiologic dead space represents the total volume of air that does not participate in gas exchange. It is the sum of anatomical dead space and alveolar dead space:
- Physiologic Dead Space = Anatomical Dead Space + Alveolar Dead Space
In healthy individuals, physiologic dead space closely approximates anatomical dead space because alveolar dead space is negligible. In patients with cardiopulmonary disease, however, physiologic dead space may increase substantially due to ventilation-perfusion mismatch.
This mismatch can occur in regions such as bronchioles, alveolar ducts, alveolar sacs, and alveoli where ventilation exceeds perfusion. As disease severity increases, physiologic dead space often rises, making it a useful indicator of impaired pulmonary function.
What Is Ventilation?
Ventilation is the mechanical process of moving air into and out of the lungs. It depends on pressure changes generated by the respiratory muscles, primarily the diaphragm and the intercostal muscles. During inhalation, the diaphragm contracts and flattens, increasing the volume of the thoracic cavity. This expansion lowers intrathoracic pressure relative to atmospheric pressure, allowing air to flow into the lungs. The external intercostal muscles assist by lifting the rib cage, further expanding the chest.
During exhalation, the diaphragm relaxes and returns to its dome-shaped position. Thoracic volume decreases, intrathoracic pressure rises, and air flows out of the lungs. At rest, exhalation is typically passive, though it can become active during exercise or respiratory distress.
Ventilation ensures a continuous supply of oxygen to the alveoli and facilitates the removal of carbon dioxide from the body. However, effective ventilation alone is not sufficient for gas exchange if perfusion is impaired.
What Is Perfusion?
Perfusion refers to the flow of blood through the pulmonary circulation. It begins when the right ventricle pumps deoxygenated blood into the pulmonary artery, which branches into progressively smaller vessels and ultimately forms capillary networks surrounding the alveoli.
Within these pulmonary capillaries, gas exchange occurs. Oxygen diffuses from the alveoli into the blood, while carbon dioxide diffuses from the blood into the alveoli. The oxygenated blood then returns to the left side of the heart, where it is pumped to the rest of the body.
Adequate perfusion is essential for effective gas exchange. Even perfectly ventilated alveoli cannot exchange gases if blood flow is absent or severely reduced. This relationship highlights the importance of ventilation-perfusion matching in maintaining normal oxygenation and carbon dioxide elimination.
Dead Space vs. Alveolar Ventilation
Understanding dead space ventilation requires distinguishing it from alveolar ventilation, which is the portion of ventilation that actually reaches functioning alveoli and contributes to gas exchange.
The relationship can be summarized as:
- Minute ventilation = tidal volume × respiratory rate
- Alveolar ventilation = (tidal volume − dead space) × respiratory rate
This means that two patients can have the same minute ventilation but very different alveolar ventilation depending on their dead space. If dead space increases, alveolar ventilation decreases unless tidal volume or respiratory rate is adjusted.
Causes of Increased Dead Space Ventilation
Increased dead space ventilation occurs when a greater proportion of inspired air fails to take part in gas exchange. This is most commonly due to impaired alveolar perfusion, structural lung abnormalities, or disruptions in pulmonary blood flow.
Conditions and factors associated with increased dead space include:
- Dysfunctional or damaged alveoli
- Decreased pulmonary perfusion
- Low cardiac output
- Hypotension
- Pulmonary vasoconstriction
- Pulmonary embolism
- Emphysema
- Pneumonia
- Acute Respiratory Distress Syndrome (ARDS)
- Increased alveolar-capillary membrane permeability
- Mechanical ventilation with endotracheal intubation
When dead space increases, ventilation becomes inefficient. More air must be moved in and out of the lungs to eliminate the same amount of carbon dioxide. This often leads to an increase in arterial carbon dioxide levels (PaCO₂) and may reduce arterial oxygen levels (PaO₂).
If compensation fails, patients may develop respiratory acidosis due to carbon dioxide retention and tissue hypoxemia due to inadequate oxygen delivery. In severe cases, progressive dead space ventilation can contribute to respiratory failure, requiring ventilatory support and aggressive management of the underlying cause.
How to Calculate Dead Space Ventilation
Physiologic dead space is commonly estimated using the Bohr equation, which relates dead space volume to tidal volume. This relationship is expressed as the dead space fraction (VD/VT):
Bohr Equation:
VD/VT = (PaCO₂ − PeCO₂) / PaCO₂
Where:
- PaCO₂ is the arterial partial pressure of carbon dioxide
- PeCO₂ is the partial pressure of carbon dioxide in mixed expired gas
Example Calculation
If a patient has a PaCO₂ of 42 mmHg and a PeCO₂ of 33 mmHg:
VD/VT = (42 − 33) / 42
VD/VT = 9 / 42
VD/VT = 0.21
This result indicates that 21% of the tidal volume is contributing to dead space ventilation. In healthy adults, a VD/VT ratio of approximately 0.20 to 0.35 is considered normal. Values above this range suggest impaired gas exchange and increased dead space, often reflecting significant cardiopulmonary pathology.
Why Dead Space Ventilation Matters in Respiratory Care
Dead space ventilation has direct implications for patient management, especially in critically ill patients and those receiving mechanical ventilation.
Impact on Carbon Dioxide Elimination
Increased dead space reduces effective alveolar ventilation, leading to carbon dioxide retention. Patients may develop hypercapnia despite apparently adequate minute ventilation.
Increased Work of Breathing
When dead space is elevated, patients must breathe faster or deeper to maintain normal CO₂ levels. This increases the work of breathing and can contribute to respiratory muscle fatigue.
Ventilator Management
Respiratory therapists must account for dead space when setting tidal volume and respiratory rate. Simply increasing respiratory rate without addressing dead space may worsen ventilation inefficiency.
Indicator of Disease Severity
An elevated VD/VT ratio is often associated with worse outcomes in conditions such as acute respiratory distress syndrome (ARDS) and severe pulmonary vascular disease. Monitoring dead space can help guide clinical decisions and assess response to therapy.
Dead Space Ventilation and Mechanical Ventilation
Mechanical ventilation can both improve and worsen dead space, depending on how it is applied.
Tidal Volume Selection
Very low tidal volumes may increase the proportion of each breath lost to anatomical dead space, especially in patients with rapid shallow breathing patterns.
PEEP and Alveolar Recruitment
Appropriate levels of PEEP can improve alveolar recruitment and reduce alveolar dead space by restoring perfusion to previously collapsed alveoli. However, excessive PEEP can overdistend alveoli and increase dead space by impairing capillary blood flow.
Circuit and Equipment Dead Space
Ventilator circuits, heat and moisture exchangers (HMEs), and inline adapters add mechanical dead space. In pediatric and neonatal patients, this added dead space can be especially significant and must be carefully managed.
Dead Space in Special Patient Populations
Pediatric and Neonatal Patients
Children and infants have smaller tidal volumes, so even small increases in dead space can significantly impair ventilation. Minimizing equipment dead space is a key responsibility for respiratory therapists in these populations.
COPD Patients
In COPD, especially emphysema, alveolar dead space is often markedly increased. These patients may require higher minute ventilation to maintain normal PaCO₂ levels and are particularly sensitive to ventilator-induced overdistention.
ARDS Patients
ARDS is associated with heterogeneous lung units, where some alveoli are collapsed and others are overdistended. This leads to increased physiologic dead space and makes ventilation strategies especially complex.
Clinical Signs of Increased Dead Space Ventilation
Respiratory therapists should be alert for signs that suggest elevated dead space, including:
- Rising PaCO₂ despite adequate minute ventilation
- Increased difference between arterial CO₂ and end-tidal CO₂
- Rapid shallow breathing
- Increased ventilatory demand and patient discomfort
- Poor response to increases in respiratory rate alone
Note: Recognizing these signs early allows for timely adjustments in therapy.
Strategies to Reduce Dead Space Ventilation
While dead space cannot be completely eliminated, several strategies can help minimize its impact:
- Optimizing tidal volume and respiratory rate
- Using appropriate PEEP to improve alveolar recruitment
- Avoiding excessive overdistention
- Reducing unnecessary equipment dead space
- Improving cardiac output and pulmonary perfusion when possible
- Treating underlying causes such as pulmonary embolism or bronchospasm
Note: These interventions require careful assessment and ongoing monitoring, a core role of respiratory therapists.
Dead Space Ventilation Practice Questions
1. What is dead space in respiratory physiology?
It is the portion of inspired air that does not participate in gas exchange.
2. Why is measuring the dead space to tidal volume (VD/VT) ratio clinically significant?
It helps assess the severity of disease and efficiency of ventilation in critically ill patients.
3. What does an increased VD/VT ratio indicate in a mechanically ventilated patient?
Reduced alveolar ventilation and impaired carbon dioxide elimination.
4. How can dead space measurements aid in ventilator weaning decisions?
They help evaluate a patient’s ability to ventilate effectively without assistance.
5. What is the equation for calculating VD/VT ratio?
(PaCO₂ – PECO₂) / PaCO₂
6. What is the normal range for the VD/VT ratio?
Typically between 0.2 and 0.4.
7. What does PaCO₂ represent?
It is the partial pressure of carbon dioxide in arterial blood.
8. What does PECO₂ represent?
It is the average partial pressure of carbon dioxide in exhaled gas.
9. Calculate the VD/VT ratio for PaCO₂ = 44 mmHg and PECO₂ = 15 mmHg.
(44 – 15) / 44 = 0.66, which indicates increased dead space.
10. What is physiologic dead space (VDphys)?
It is the total volume of air that does not participate in gas exchange, including anatomic and alveolar dead space.
11. What should physiologic dead space normally equal?
Anatomic dead space, if no alveolar dead space is present.
12. What is the equation for physiologic dead space (VDphys)?
[(PaCO₂ – PECO₂) / PaCO₂] × VT
13. What is anatomic dead space?
Air occupying the conducting airways that does not reach the alveoli for gas exchange.
14. How is anatomic dead space estimated?
Approximately 1 mL per pound of ideal body weight.
15. What is alveolar dead space?
Ventilated alveoli that are not perfused, preventing gas exchange.
16. What is the normal alveolar dead space in a healthy individual?
Zero or minimal.
17. Calculate VD/VT and VDphys for PaCO₂ = 42 mmHg, PECO₂ = 32 mmHg, and VT = 560 mL.
VD/VT = 0.24; VDphys = 0.24 × 560 = 134 mL
18. What are the two components of physiologic dead space?
Anatomic dead space and alveolar dead space.
19. How do you calculate alveolar dead space?
VDphys – VDanatomic
20. Solve for VD/VT, VDphys, VDanatomic, and VDalv for: PaCO₂ = 49 mmHg, PECO₂ = 28 mmHg, VT = 575 mL, IBW = 125 lb.
VD/VT = 0.43; VDphys = 247 mL; VDanatomic = 125 mL; VDalv = 122 mL
21. What does VA represent in pulmonary physiology?
Alveolar ventilation—the portion of inspired air that reaches alveoli and participates in gas exchange.
22. What is the equation for alveolar ventilation (VA)?
VA = VT – VDphys
23. What is dead space ventilation?
Ventilation without perfusion, resulting in wasted ventilation.
24. List the three types of dead space in respiratory physiology.
Anatomic dead space, alveolar dead space, and physiologic dead space.
25. What clinical conditions can cause increased alveolar dead space?
Pulmonary embolism, low cardiac output, and severe hypotension.
26. What part of the respiratory tract makes up the anatomic dead space?
The conducting airways from the nose and mouth to the terminal bronchioles where no gas exchange occurs.
27. Approximately how much of each tidal volume is considered anatomic dead space?
About one-third (1/3) of the tidal volume.
28. When does the VD/VT ratio increase?
When tidal volume (VT) decreases.
29. What defines alveolar dead space?
Lung volume that cannot participate in gas exchange due to reduced or absent pulmonary perfusion, such as in a pulmonary embolism.
30. What is physiologic dead space?
The sum of anatomic and alveolar dead space.
31. What is the most accurate measurement of ventilatory dead space?
Physiologic dead space.
32. What is the formula for calculating physiologic dead space?
Anatomic dead space + Alveolar dead space.
33. What happens to tidal volume when physiologic dead space increases?
Tidal volume decreases.
34. What occurs when tidal volume decreases in the presence of increased physiologic dead space?
There is a relative increase in the VD/VT ratio, often seen in drug overdose or neuromuscular disease.
35. What does an increase in physiological dead space imply about alveolar dead space?
Alveolar dead space is also increased.
36. What conditions can increase alveolar dead space due to decreased cardiac output?
Congestive heart failure and blood loss.
37. What conditions can increase alveolar dead space due to pulmonary vessel obstruction?
Pulmonary vasoconstriction and pulmonary embolism.
38. What is the normal VD/VT ratio?
25–35%.
39. What is the formula to calculate the VD/VT ratio?
(PaCO₂ – PECO₂) / PaCO₂
40. What does a VD/VT ratio greater than 60% suggest?
Impending ventilatory failure.
41. What can a persistent increase in physiologic VD/VT lead to?
An ongoing increase in the work of breathing (WOB).
42. What may occur if a patient cannot sustain an increased WOB due to elevated VD/VT?
Ventilatory and oxygenation failure may occur.
43. What is dead space ventilation?
The volume of inhaled air that does not participate in gas exchange.
44. What does anatomic dead space include?
The area from the nose and mouth down to the terminal bronchioles.
45. What defines alveolar dead space?
Ventilated alveoli that are not perfused with blood.
46. What is a common clinical cause of increased alveolar dead space?
A pulmonary embolus.
47. True or False: Anatomic dead space is a normal and expected finding.
True
48. True or False: Alveolar dead space is abnormal because alveoli should be perfused.
True
49. What is a primary cause of alveolar dead space?
A pulmonary embolus that obstructs blood flow to a segment of the lung.
50. What condition leads to increased alveolar dead space by impairing perfusion?
Pulmonary embolism.
51. What structures are included in anatomic dead space?
The trachea, pharynx, larynx, bronchi, and nasal passages.
52. What does dead space ventilation refer to?
The volume of air remaining in the conducting airways that does not reach the alveoli.
53. What is the definition of dead space?
Ventilation without perfusion, also known as wasted ventilation.
54. What are the three types of dead space?
Anatomic, alveolar, and physiologic.
55. What is the definition of anatomic dead space?
The volume of air left in the conducting airways during a breath that does not reach the alveoli.
56. What is a primary cause of alveolar dead space?
A pulmonary embolism or decreased cardiac output.
57. How is physiologic dead space defined?
It is the sum of anatomic dead space and alveolar dead space.
58. How do you estimate anatomic dead space?
1 mL per pound of ideal body weight.
59. What formula is used to calculate ideal body weight for men?
50 + (0.91 × [height in cm − 152.4]).
60. What formula is used to calculate ideal body weight for women?
45.5 + (0.91 × [height in cm − 152.4]).
61. How do you calculate dead space as a percentage?
(PaCO₂ – PECO₂) / PaCO₂.
62. Why is the PO₂ of tracheal gas less than that of atmospheric gas?
Because tracheal gas includes water vapor pressure, reducing the available pressure for oxygen.
63. What happens to VA and VDanat when VT increases?
VDanat remains constant, while VA increases.
64. If dead space increases while VE remains the same, what happens to VA and gas exchange?
VA decreases, leading to reduced gas exchange efficiency and increased PaCO₂.
65. If VT = 600 mL, f = 10 bpm, PaCO₂ = 40 mmHg, and VCO₂ = 200 mL/min, what is the VD?
VD = 1685 mL/min after calculating VE – VA.
66. A patient has a VE of 18 L/min and a PaCO₂ of 40 mmHg. What does this indicate?
Increased dead space, likely due to conditions like pulmonary embolism or hypotension.
67. What is the general definition of dead space?
The volume of the airways that does not take part in gas exchange.
68. Where does anatomic dead space end in the respiratory system?
At generation 16, the terminal bronchioles.
69. How many generations make up the tracheobronchial tree?
23 generations in total.
70. What generations make up the conducting zone?
Generations 0–16.
71. What generations make up the respiratory zone?
Generations 17–23.
72. What is alveolar dead space?
The volume of alveoli that are ventilated but not perfused.
73. What is physiologic dead space?
The sum of anatomic and alveolar dead space.
74. What factors can increase dead space?
Lung volume, bronchodilation, neck extension, pulmonary embolism, age, hypotension, hemorrhage, pulmonary disease, general anesthesia, IPPV, atropine, and hyoscine.
75. How does increased lung volume affect dead space?
It increases anatomical dead space by enlarging the conducting airways.
76. How does bronchodilation influence dead space?
It increases anatomical dead space by expanding airway diameter.
77. How does a pulmonary embolism increase dead space?
It increases alveolar dead space by reducing pulmonary perfusion to ventilated alveoli.
78. How does pulmonary disease contribute to increased dead space?
It alters diffusion at the alveolar-capillary membrane, increasing alveolar dead space.
79. How can general anesthesia increase dead space?
It increases anatomical dead space through bronchodilation and may impair perfusion through hypotension.
80. How do PEEP and IPPV contribute to increased dead space?
They increase anatomical dead space by expanding lung volume and increase alveolar dead space by reducing pulmonary blood flow.
81. How does atropine or hyoscine affect dead space?
They increase anatomical dead space due to bronchodilation.
82. What interventions can decrease dead space?
Tracheal intubation, tracheostomy, and positioning the patient supine.
83. What is Fowler’s method used to measure?
Anatomic dead space using the single-breath nitrogen washout technique.
84. What gas is analyzed in Fowler’s method during exhalation?
Nitrogen concentration is analyzed to assess changes in expired gas composition.
85. What occurs during Phase 1 of Fowler’s method?
Exhaled gas comes entirely from anatomic dead space, so no nitrogen is present.
86. What occurs during Phase 2 of Fowler’s method?
A mixture of dead space and alveolar gas results in a rising nitrogen concentration.
87. What is observed in Phase 3 of Fowler’s method?
An alveolar plateau with mixed gas from upper and lower lung regions.
88. What causes Phase 4 in Fowler’s method?
Closure of lower airways leads to exhalation of nitrogen-rich gas from upper airways.
89. What is the normal value of anatomic dead space?
Approximately 2 mL/kg or 150 mL in a healthy adult.
90. What is closing capacity (CC)?
The lung volume at which airway closure begins, calculated as CC = CV + RV.
91. What factors increase closing capacity?
Age, supine position, anesthesia, increased intrathoracic pressure, and smoking.
92. When does closing capacity approach or exceed FRC?
In conditions like obesity, supine positioning, and under anesthesia.
93. What equation is used to measure physiological dead space?
The Bohr equation: Vd/Vt = (PaCO₂ – PeCO₂) / PaCO₂.
94. What principle underlies the Bohr equation?
It compares CO₂ levels in arterial and exhaled air to estimate ventilated but non-perfused volume.
95. Does all inspired air reach the alveoli for gas exchange?
No, due to the presence of dead space.
96. What is physiologic dead space?
The total volume of inspired air that does not participate in gas exchange.
97. What is anatomic dead space?
The volume of air in the conducting zone that does not reach the alveoli.
98. What is alveolar dead space?
The volume of air in alveoli that are ventilated but not perfused.
99. What two components make up physiologic dead space?
Anatomic dead space + alveolar dead space.
100. In healthy individuals, what should physiologic dead space equal?
It should be equal to the anatomic dead space.
Final Thoughts
Dead space ventilation is more than a theoretical concept; it is a practical and clinically significant factor that directly influences gas exchange, ventilator management, and patient outcomes.
For respiratory therapists, understanding the types of dead space, recognizing conditions that increase it, and knowing how to respond are essential skills.
By carefully evaluating ventilation efficiency and adjusting therapy accordingly, respiratory therapists play a crucial role in reducing ventilatory inefficiency, easing the work of breathing, and supporting effective respiratory care across a wide range of clinical settings.
Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
References
- Intagliata S, Rizzo A, Gossman W. Physiology, Lung Dead Space. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025.