Lung adenocarcinoma is the most common type of lung cancer, accounting for a significant portion of cases worldwide. Unlike some other forms of lung cancer, it often develops in the outer regions of the lungs and is closely linked to both smoking and non-smoking risk factors, making it a concern for a wide range of individuals.
Because it tends to grow and spread silently in its early stages, many people are unaware they have the disease until it becomes more advanced.
Recognizing the potential causes, early warning signs, and available treatment options is essential for improving outcomes and offering patients the best chance at recovery.
Download our free guide that has over 100+ of the best tips for healthy lungs.
What is Lung Adenocarcinoma?
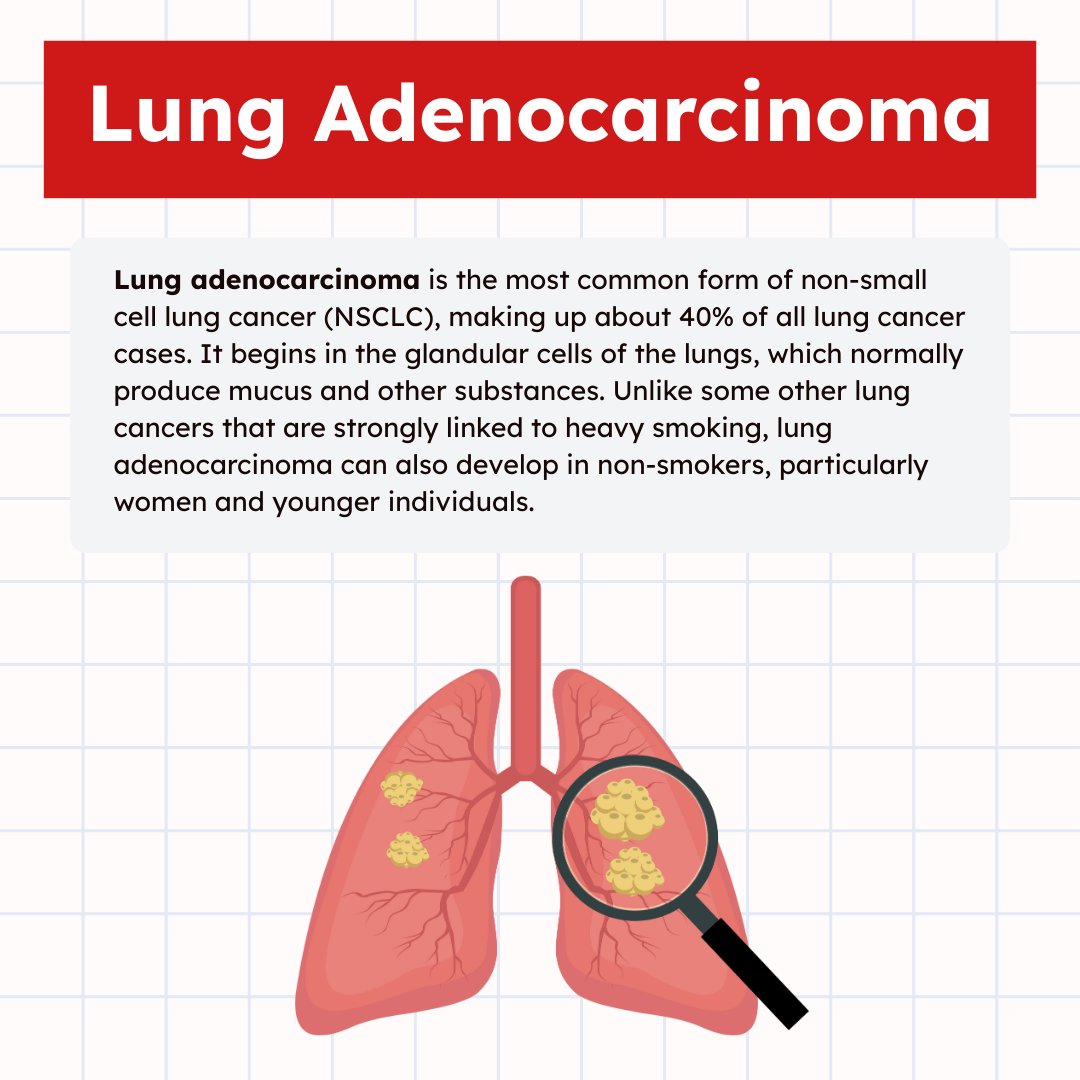
Lung adenocarcinoma is the most common form of non-small cell lung cancer (NSCLC), making up about 40% of all lung cancer cases. It begins in the glandular cells of the lungs, which normally produce mucus and other substances. Unlike some other lung cancers that are strongly linked to heavy smoking, lung adenocarcinoma can also develop in non-smokers, particularly women and younger individuals.
This type of cancer typically forms in the outer parts of the lungs and often grows more slowly than other lung cancers, which may allow for earlier detection in some cases. However, it can spread to lymph nodes and distant organs if left untreated, making early recognition and treatment essential for better outcomes.
Epidemiology
Lung adenocarcinoma accounts for approximately 40% of all lung cancer cases worldwide. It has increased in incidence relative to other lung cancer types, partly due to changing smoking habits and improved detection methods.
It predominates in adults over 45 and is the leading lung cancer subtype among never-smokers. Geographically, rates are highest in developed countries, correlating with environmental exposures and diagnostic access.
Men and women are both affected, but a growing proportion of cases occur in females, reflecting shifts in risk profiles. Survival rates vary depending on stage at diagnosis and treatment but generally remain low in advanced disease.
Risk Factors
The primary risk factor for lung adenocarcinoma remains tobacco smoking, but it develops more frequently than other lung cancers in never-smokers. Exposure to secondhand smoke also increases risk.
Other recognized factors include long-term exposure to radon gas, asbestos, diesel exhaust, and air pollution. Genetic mutations like EGFR and ALK rearrangements contribute to tumor development, particularly in non-smokers.
Chronic lung diseases, such as pulmonary fibrosis, and a history of prior cancer treatment can elevate risk. Family history and certain ethnic backgrounds may also affect susceptibility.
Classification
Lung adenocarcinoma is classified based on histological growth patterns and cellular differentiation. Major patterns include lepidic, acinar, papillary, micropapillary, and solid types, each linked to prognosis.
The 2015 WHO classification emphasizes invasive versus non-invasive components, impacting treatment decisions. Tumors with predominant lepidic patterns tend to have better outcomes.
Molecular classification has become critical, identifying mutations in genes such as EGFR, KRAS, and ALK. These molecular profiles guide targeted therapies and influence prognosis.
Pathologists use morphology and biomarker testing to assign subtypes, essential for personalized patient management.
Pathogenesis and Molecular Biology
Lung adenocarcinoma develops through complex genetic and molecular changes influenced by various internal and external factors. Several key molecular drivers and interactions within the tumor environment contribute to its initiation and progression.
Genetic Alterations
Mutations in the EGFR, KRAS, and ALK genes are among the most frequent genetic changes in lung adenocarcinoma. EGFR mutations often lead to uncontrolled cell proliferation by activating signaling pathways such as PI3K/AKT and RAS/RAF/MEK. In contrast, KRAS mutations are associated with resistance to certain targeted therapies and are linked to poorer outcomes.
Other alterations include BRAF, MET, and HER2 mutations, as well as rearrangements in ROS1 and RET. These mutations provide potential targets for personalized treatment. Tumor suppressor genes such as TP53 and STK11 also exhibit frequent inactivation, which contributes to genomic instability and cancer progression.
Molecular Subtypes
Lung adenocarcinoma is categorized into molecular subtypes based on dominant driver mutations, affecting prognosis and treatment options. Subtypes include EGFR-mutated, ALK-rearranged, KRAS-mutated, and wild-type, each with unique molecular profiles and clinical features.
Targeted therapies have been developed for specific subtypes. For example, tyrosine kinase inhibitors target EGFR mutations and ALK fusions. The efficacy of immunotherapy varies among subtypes, often influenced by tumor mutational burden and PD-L1 expression, which are important considerations for treatment planning.
Role of Smoking
Smoking is a strong risk factor, contributing to DNA damage and mutation accumulation. It is particularly linked to KRAS mutations, which are more common in smokers than non-smokers. Tobacco carcinogens induce a mutational signature characterized by G to T transversions.
Non-smokers with lung adenocarcinoma often carry EGFR mutations or ALK rearrangements, indicating distinct molecular pathways driven by environmental and genetic factors. Smoke exposure also affects the tumor microenvironment and immune response, influencing tumor behavior.
Tumor Microenvironment
The tumor microenvironment consists of stromal cells, immune cells, blood vessels, and extracellular matrix components interacting with cancer cells. It plays a critical role in tumor growth, metastasis, and therapy resistance.
Immune cells such as tumor-associated macrophages can create an immunosuppressive environment, reducing the effectiveness of immune checkpoint inhibitors. Angiogenesis driven by vascular endothelial growth factor supports tumor survival and progression. Understanding these interactions is crucial for developing combination therapies.
Clinical Presentation
Lung adenocarcinoma typically presents with a range of clinical features depending on tumor size, location, and extent of spread. Early stages may be asymptomatic, while advanced disease often involves more pronounced respiratory and systemic signs.
Signs and Symptoms
Patients often report a persistent cough that may progress over weeks or months. Hemoptysis (coughing up blood) occurs in some cases but is less common than with squamous cell carcinoma. Chest pain can result from tumor invasion into the pleura or chest wall.
Other symptoms include shortness of breath, wheezing, and recurrent respiratory infections. Constitutional symptoms such as weight loss, fatigue, and anorexia frequently appear in advanced stages. Paraneoplastic syndromes, although rare, may cause endocrine or neurologic symptoms.
Staging
Staging follows the TNM system (Tumor, Node, Metastasis) and is crucial for prognosis and treatment planning. Early-stage lung adenocarcinoma (stage I) is confined to the lung with no lymph node involvement.
Stages II and III involve increasing tumor size and regional lymph node spread. Stage IV indicates distant metastases, commonly to the brain, bones, liver, or adrenal glands. Imaging studies and biopsy confirm the extent of disease.
Differential Diagnosis
Differential diagnosis includes other primary lung cancers, such as squamous cell carcinoma and small cell lung cancer. Infectious diseases like tuberculosis and fungal infections can mimic local lesions or mass effects.
Benign lung nodules and metastatic tumors from other organs should also be considered. Radiologic findings combined with histopathologic examination are essential to differentiate lung adenocarcinoma from these conditions.
Diagnostic Methods
Lung adenocarcinoma diagnosis relies on a combination of imaging, tissue sampling, microscopic examination, and molecular testing. Each method provides specific information crucial for confirming the presence of cancer and guiding treatment decisions.
Imaging Techniques
Imaging is the first step in detecting lung adenocarcinoma. Chest X-rays can reveal abnormal masses but have limited detail. Computed tomography (CT) scans offer a detailed view of lung nodules, size, shape, and lymph node involvement. CT scans help distinguish between benign and malignant lesions more effectively than X-rays.
Positron emission tomography (PET) scans detect metabolic activity of tumors, aiding in staging and identifying metastasis. PET-CT combines anatomical and metabolic data for precise assessment. MRI is less common but useful for assessing brain metastasis if symptoms suggest spread.
Biopsy Procedures
Biopsy confirms a tissue diagnosis. Common methods include bronchoscopy, which allows direct visualization and biopsy of central lung lesions. CT-guided needle biopsy is preferred for peripheral nodules. It involves inserting a needle through the chest wall into the tumor, guided by CT images.
Thoracentesis or pleural biopsy may be performed if fluid accumulates around the lungs. Surgical biopsies, like video-assisted thoracoscopic surgery (VATS), are reserved for cases where less invasive methods fail.
Histopathology
Histopathological examination identifies the cellular characteristics of lung adenocarcinoma. Tissue samples are stained and examined under a microscope to detect malignant glandular cells. The pattern of growth, mucin production, and cellular differentiation help distinguish adenocarcinoma from other lung cancers.
Immunohistochemistry (IHC) further confirms the diagnosis by detecting specific proteins such as TTF-1 and Napsin A, markers commonly expressed in lung adenocarcinoma cells.
Note: Accurate histopathology guides treatment choice and prognosis estimation.
Biomarker Testing
Molecular biomarker testing detects genetic mutations and alterations driving tumor growth. Common targets include EGFR mutations, ALK and ROS1 rearrangements, KRAS, and BRAF mutations.
Testing is usually performed on biopsy tissue or sometimes a liquid biopsy from blood. Biomarker results determine eligibility for targeted therapies or immunotherapies. Routine testing for PD-L1 expression also assesses potential response to checkpoint inhibitors. This personalized approach improves treatment precision and outcomes.
Treatment Approaches
Treatment for lung adenocarcinoma varies depending on the stage, genetic mutations, and patient health. Options include surgery to remove tumors, targeted drugs for specific mutations, immune checkpoint inhibitors, and radiation to control tumor growth or relieve symptoms.
Surgical Management
Surgery is the preferred option for early-stage lung adenocarcinoma when the tumor is localized and the patient can tolerate the procedure. Common surgeries include lobectomy (removal of a lung lobe), segmentectomy, or pneumonectomy in more extensive cases.
Surgical removal aims to achieve clear margins, meaning no cancer cells remain at the edges of removed tissue. Lymph node dissection is often performed to assess cancer spread. Surgery may be combined with chemotherapy or radiation if there is a risk of microscopic residual disease.
Targeted Therapy
Targeted therapy focuses on genetic mutations such as EGFR, ALK, ROS1, and BRAF that drive cancer growth. Drugs like osimertinib target EGFR mutations, while crizotinib and alectinib are used for ALK-positive tumors.
These treatments are oral medications designed to inhibit cancer-specific proteins. They typically have fewer side effects than chemotherapy but require genetic testing of the tumor before use. Resistance can develop, requiring alternative therapies or combination approaches.
Immunotherapy
Immunotherapy activates the body’s immune system to attack cancer cells. Checkpoint inhibitors such as pembrolizumab and nivolumab block PD-1/PD-L1 pathways, allowing immune cells to recognize and kill tumor cells.
This approach is effective, especially in tumors with high PD-L1 expression. It can be used alone or combined with chemotherapy, depending on cancer stage and patient factors. Side effects include immune-related inflammation in organs, which requires monitoring.
Radiation Therapy
Radiation therapy uses high-energy rays to destroy cancer cells or shrink tumors. It is often used when surgery is not possible or to treat metastases and relieve symptoms like pain or airway obstruction.
Techniques include external beam radiation, stereotactic body radiotherapy (SBRT) for early, inoperable tumors, and palliative radiation. Radiation can be combined with chemotherapy or immunotherapy to improve outcomes. Side effects depend on the treated area and dose.
Prognosis and Survival
The outcome for patients with lung adenocarcinoma depends on several clinical and biological factors. Survival varies widely based on disease stage, treatment response, and patient health.
Prognostic Factors
Tumor stage at diagnosis is the most critical factor influencing prognosis. Early-stage tumors confined to the lungs generally have better outcomes, while metastatic disease significantly lowers survival chances.
Other important factors include tumor size, lymph node involvement, and molecular characteristics such as EGFR or ALK mutations. These genetic alterations can guide targeted therapies and impact survival positively.
Patient-related factors like age, overall health, and smoking history also affect prognosis. Performance status, measured by scales such as ECOG, helps predict treatment tolerance and outcome.
Survival Rates
Survival rates for lung adenocarcinoma vary based on stage and treatment. The 5-year survival rate for localized disease can reach approximately 60%, whereas advanced metastatic cases have rates below 10%.
Targeted therapies and immunotherapies have improved survival in patients with specific mutations or biomarkers. However, survival remains limited in late-stage disease despite advances.
Median survival time for advanced lung adenocarcinoma is often measured in months without effective treatment. Early detection remains crucial for improving survival chances.
Monitoring and Follow-Up
Regular monitoring after initial treatment is essential to detect recurrence or progression early. Follow-up typically involves imaging studies such as CT scans every 3 to 6 months in the first few years.
Blood tests and assessment of symptoms also guide clinical decisions during follow-up. Patients with actionable mutations require ongoing evaluation for resistance to targeted therapies.
Long-term follow-up aims to manage side effects of treatment and maintain quality of life. Coordination between oncologists, radiologists, and primary care providers supports optimal surveillance.
Prevention and Future Directions
Preventing lung adenocarcinoma relies heavily on reducing risk factors and early detection. Future treatment advancements aim to target molecular pathways and improve survival rates. Ongoing research focuses on identifying biomarkers and refining personalized therapy.
Prevention Strategies
The primary prevention approach is eliminating tobacco exposure, as smoking causes most lung adenocarcinomas. Avoiding secondhand smoke and promoting smoking cessation programs are critical public health measures.
Environmental factors, such as reducing indoor radon and air pollution exposure, contribute to lowering risk. Routine screenings with low-dose computed tomography (LDCT) in high-risk populations improve early diagnosis.
Vaccination against human papillomavirus (HPV) may also reduce lung cancer risk, as some studies show links between HPV infection and lung adenocarcinoma development. Combined lifestyle changes and environmental controls remain foundational for prevention.
Emerging Therapies
Targeted therapies have transformed lung adenocarcinoma treatment, focusing on specific mutations like EGFR, ALK, and ROS1. Tyrosine kinase inhibitors (TKIs) provide improved outcomes over traditional chemotherapy.
Immunotherapy, particularly immune checkpoint inhibitors targeting PD-1/PD-L1, shows promising results by enhancing the body’s immune response against cancer cells. Combination treatments integrating immunotherapy and TKIs are under evaluation.
Novel agents affecting angiogenesis, MET inhibitors, and antibody-drug conjugates expand options. Personalized treatment based on molecular profiling is increasingly standard to maximize efficacy and reduce toxicity.
Research Trends
Research increasingly targets early detection methods, such as liquid biopsies analyzing circulating tumor DNA for non-invasive diagnostics. This approach aims to catch lung adenocarcinoma at treatable stages.
Genomic and proteomic studies identify new biomarkers and resistance mechanisms to existing therapies, aiding in treatment customization. Efforts explore the tumor microenvironment’s role in metastasis and immune evasion.
Clinical trials focus on combination therapies and novel immune modulators to overcome resistance. Large-scale data integration and artificial intelligence assist in predicting patient responses and optimizing care.
Final Thoughts
Lung adenocarcinoma remains a serious health challenge, but advances in research and treatment are providing hope for patients and families. By understanding the causes, recognizing early signs, and seeking prompt medical care, individuals can improve their chances of successful treatment.
Options such as surgery, chemotherapy, targeted therapy, immunotherapy, and radiation continue to evolve, offering more personalized approaches to care.
While prevention through lifestyle choices, such as avoiding smoking and reducing environmental risks, remains vital, ongoing medical advancements are helping to turn the tide against this disease. Greater awareness and early detection are crucial steps toward achieving better outcomes and an improved quality of life for those affected.
Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
References
- Clark SB, Alsubait S. Non–Small Cell Lung Cancer. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025.
- Myers DJ, Wallen JM. Lung Adenocarcinoma. [Updated 2023 Jun 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025.