For individuals living with severe COPD, particularly emphysema, even basic activities like walking or climbing stairs can become a daily struggle. As the disease progresses, trapped air in damaged lungs causes hyperinflation, making it increasingly difficult to breathe.
The Zephyr Valve is a groundbreaking, minimally invasive treatment option designed to address this issue by improving lung function without surgery.
By strategically placing tiny one-way valves in the airways, this therapy allows the healthier parts of the lungs to expand and function more efficiently. In this article, we’ll explore the purpose, benefits, and inner workings of the Zephyr Valve for COPD management.
Download our free guide that has over 100+ of the best tips for healthy lungs.
What Is the Zephyr Valve?
The Zephyr Valve is a minimally invasive device designed to help people with severe emphysema breathe more easily. It works by reducing lung hyperinflation and improving airflow for better lung function.
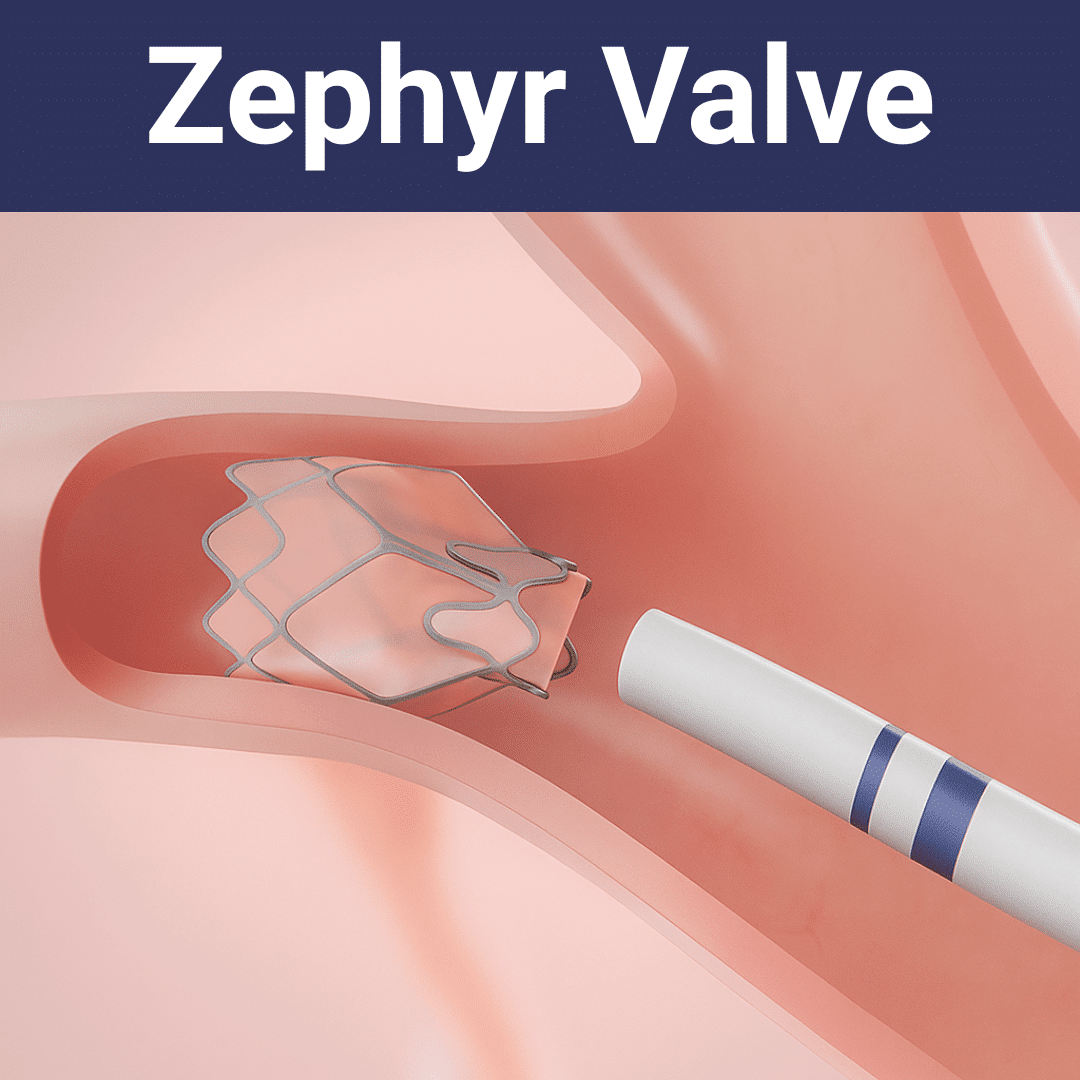
Overview of Design and Function
The Zephyr Valve is a tiny, one-way valve made from medical-grade materials, including nitinol and silicone. It is implanted using a bronchoscope, which is a thin tube inserted through the mouth or nose and into the airways.
Each valve is placed in the airways of the lung’s most damaged areas. Once in position, it allows trapped air and gases to escape from the affected part of the lung during exhalation, but stops any new air from entering those sections. This reduces the volume of the diseased lung areas and gives the healthier lung tissue more room to expand with each breath.
Note: The procedure is performed under moderate sedation and lasts about one hour. Most patients do not require incisions or general anesthesia.
Key Features
The Zephyr Valve is approved by the FDA for adults with severe emphysema who do not respond well to other treatments. It is removable and does not permanently alter lung tissue.
The valve is usually well tolerated, with most patients experiencing immediate effects on breathing. The device is designed to minimize disruption to healthy lung areas and is adjustable if repositioning becomes necessary.
Risks can include pneumonia, pneumothorax (collapsed lung), or airway irritation, but these are monitored closely by medical teams. The Zephyr Valve is only offered at specialized medical centers with experience in advanced lung disease interventions.
How the Zephyr Valve Works
The Zephyr Valve is a small, one-way valve designed to treat severe emphysema by improving lung function. Its success relies on a minimally invasive placement procedure and specific mechanisms that target damaged lung areas without the need for open surgery.
Bronchoscopic Placement
Doctors place the Zephyr Valve using a flexible bronchoscope, which is a thin, tube-like instrument inserted through the mouth or nose. The procedure is performed under moderate sedation or general anesthesia in a hospital setting.
The bronchoscope allows direct visualization of the airways, guiding the placement of the valves into the targeted airways in the diseased part of the lung. Usually, a pulmonologist carefully measures which airways to target by assessing lung imaging and airflow studies before the procedure.
Each valve is inserted to block airflow to the most damaged lung segments. On average, three to five valves are placed during each procedure. Once installed, the bronchoscope is carefully withdrawn, and the patient is monitored for complications such as pneumothorax or infection.
Mechanisms of Action
The Zephyr Valve permits trapped air and fluids to exit the diseased part of the lung, but it prevents new air from entering those segments. This one-way action is achieved with a duckbill-shaped mechanism made from a combination of silicone and nickel-titanium alloys.
By blocking airflow into hyperinflated or poorly functioning lung areas, the valves cause these regions to deflate. This reduces lung volume in diseased segments, allowing healthier portions of the lung to expand and function more efficiently.
The main benefits include easier breathing, increased exercise tolerance, and reduced shortness of breath. Regular follow-ups and pulmonary function tests assess the continued effectiveness of the valves and monitor for potential side effects.
Zephyr Valve Indications
Zephyr Valves are designed for the treatment of severe emphysema that is not well managed with medication. They are intended for carefully selected patients who meet specific medical criteria.
Emphysema and COPD Eligibility
Zephyr Valves are indicated for adults with severe emphysema, a form of chronic obstructive pulmonary disease (COPD). Eligible patients typically have hyperinflation of the lungs, with persistent shortness of breath even after optimal medical therapy.
Patients must have evidence of heterogeneous emphysema, where some lobes are more damaged than others. Pulmonary function tests usually show an FEV1 (forced expiratory volume in one second) between 15% and 50% of the predicted value. Imaging, such as high-resolution CT scans, is used to confirm suitability and lung anatomy.
Note: The device is not suitable for all COPD patients. Those with significant collateral ventilation—the movement of air between lung segments—are typically excluded, as the valves will not function as intended in these cases.
Suitability Criteria
To qualify for Zephyr Valve implantation, several medical criteria must be met. Candidates should have a stable condition without frequent recent exacerbations or severe coexisting conditions that raise surgical risks.
A thorough clinical assessment confirms that conventional treatments have failed to control symptoms. Patients must also be able to undergo bronchoscopy, the procedure needed for valve placement.
Certain factors, such as active respiratory infections, severe bronchiectasis in the target lobe, or an inability to walk, may disqualify someone from the procedure. Candidates need thorough pre-procedural evaluation, including tests for collateral ventilation, to ensure the best outcomes.
Zephyr Valve Procedure Details
This procedure requires careful patient selection and a series of well-defined steps. Clinical considerations are important to maximize the safety and benefit of the intervention.
Pre-Procedure Assessment
Patients undergo a comprehensive pulmonary evaluation before the Zephyr Valve procedure. This typically includes spirometry, lung volume measurements, and imaging studies such as high-resolution CT scans to assess emphysema distribution.
Physicians also review the patient’s medical history, focusing on prior lung surgeries, infections, and other health conditions that could impact recovery. Screening checks often include blood tests and an arterial blood gas analysis.
A six-minute walk test is commonly performed to evaluate exercise tolerance and oxygen requirements. Patients must meet certain criteria regarding lung function, air trapping, and lack of collateral ventilation to qualify for the valve placement.
Procedure Steps
The Zephyr Valve procedure is performed using bronchoscopy under sedation or general anesthesia. The clinician navigates a thin, flexible bronchoscope into the airways and identifies target lobes for treatment.
One or more Zephyr Valves are carefully placed via the bronchoscope into the small passages of the diseased lung lobe. These valves are designed to block airflow into the lobe while allowing trapped air and secretions to escape.
Placement usually takes less than an hour. After valve insertion, the patient is observed and monitored for complications, such as pneumothorax or infection, often staying in the hospital for a few days for recovery and follow-up assessments.
Benefits of the Zephyr Valve
Zephyr Valve is a minimally invasive option that addresses symptoms and lung function in selected patients with severe emphysema. Its design allows for targeted airflow management, offering important relief without surgery.
Symptom Relief
The primary benefit of the Zephyr Valve is reduced shortness of breath. Many patients report significant improvement in breathing within weeks after the procedure.
By blocking airflow to diseased lung sections, trapped air is released and neighboring healthy tissue can expand. This change often translates to less daily fatigue, making walking and basic activities easier.
Patients frequently experience improved exercise tolerance. According to clinical studies, much of the discomfort from hyperinflation decreases, and many notice less wheezing and coughing.
Individuals with the Zephyr Valve often rely less on rescue inhalers or nebulizers for flare-ups. They can perform daily activities—like dressing or climbing stairs—with fewer interruptions due to breathlessness.
Improved Lung Function
Lung function tests, such as FEV1 (forced expiratory volume in one second), often show measurable gains after valve placement. This is because the Zephyr Valve improves the efficiency of the remaining healthy lung by releasing trapped air from emphysematous regions.
Oxygen levels in some patients can increase, and the overall work of breathing is reduced. This mechanical change can allow for lower supplemental oxygen needs or less time spent on oxygen therapy, depending on pre-existing requirements.
Patients often report greater ease in performing moderate tasks, such as household chores or light walking. These improvements in measurable lung function are generally maintained for at least several months after the procedure, based on trial data.
Note: Structural changes to the lung can also reduce risk of certain complications, such as recurrent lung infections due to stagnant air, though careful monitoring and follow-up remain essential.
Risks and Complications
The Zephyr Valve is generally well-tolerated, but potential risks should be considered before treatment. Some patients may experience complications related to the procedure or the device itself.
Common risks include:
- Pneumothorax (collapsed lung), particularly in the days following valve placement
- Chest discomfort or pain
- Mild bleeding during or after the procedure
- Shortness of breath
Rarely, patients may have an infection, including pneumonia or bronchitis. Respiratory infections can require antibiotics or, in some cases, removal of the valve.
A small number of patients may need the valve removed if it causes significant problems or does not improve symptoms. Less commonly, the valve may shift position or become blocked by mucus.
Patients should report new or worsening symptoms to their healthcare provider promptly. The risks and benefits should always be discussed with a medical team before deciding on the Zephyr Valve.
Pros and Cons of the Zephyr Valve
The Zephyr Valve is a breakthrough treatment for patients with advanced COPD and emphysema who are no longer finding relief from medication or lifestyle changes. By offering a minimally invasive alternative to surgery, it helps improve breathing by targeting and isolating damaged areas of the lungs.
However, like any medical intervention, it comes with its own set of advantages and potential drawbacks. Below is a detailed look at the pros and cons to help patients make informed decisions with their healthcare provider.
Pros of the Zephyr Valve
- Minimally Invasive Procedure: Unlike traditional lung volume reduction surgery, the Zephyr Valve is inserted using a bronchoscope, meaning no incisions, stitches, or major surgical recovery are required. This minimally invasive approach significantly reduces hospital stay, postoperative discomfort, and the risk of complications, making it a more appealing option for many patients with advanced COPD.
- Improved Lung Function and Breathing: The valve works by blocking airflow to the most damaged areas of the lungs, allowing healthier regions to expand and function more effectively. This reduces lung hyperinflation, eases pressure on the diaphragm, and results in noticeably improved breathing. Many patients experience a reduction in shortness of breath and can perform physical activities with greater ease.
- Enhanced Quality of Life: Patients often report a substantial improvement in their overall well-being following Zephyr Valve placement. Improved respiratory function translates into increased mobility, reduced fatigue, and greater independence in daily activities. For many, the procedure restores a level of activity and engagement they hadn’t experienced in years.
Cons of the Zephyr Valve
- Potential Complications: While the Zephyr Valve is considered safe, it is not without risks. Some patients may experience a temporary worsening of symptoms or side effects such as wheezing, increased mucus production, or even pneumonia. These complications are typically manageable but may require close medical follow-up or hospitalization.
- Not Suitable for Everyone: The effectiveness of the Zephyr Valve depends heavily on specific lung characteristics. It’s most beneficial for patients with severe emphysema and minimal collateral ventilation between lung lobes. Those who don’t meet the criteria may not see meaningful improvement and may even be ineligible for the procedure after evaluation.
- Cost and Insurance Limitations: Cost can be a barrier for many individuals considering this treatment. Although the Zephyr Valve is FDA-approved and covered by some insurance providers, coverage varies by plan. Patients may face high out-of-pocket costs if their insurance doesn’t fully cover the procedure. A thorough discussion with the provider and insurance company is essential before moving forward.
Recovery and Aftercare
Patients typically recover in the hospital for a short period following Zephyr Valve placement. Careful monitoring and structured follow-up appointments help ensure the best possible outcome and reduce the risk of complications.
Hospital Stay and Monitoring
After the Zephyr Valve procedure, most patients remain in the hospital for three to five days. During this time, medical staff observe for signs of complications such as pneumothorax (collapsed lung), infection, or bleeding.
Regular chest X-rays and vital sign checks are standard practice to monitor lung function. Oxygen levels are assessed frequently, and breathing exercises may be recommended to support lung recovery.
Patients may experience some coughing, mild chest discomfort, or shortness of breath, which is typically managed with medication as needed. Discharge depends on the patient’s stability and the absence of serious complications.
Follow-Up Care
Post-discharge, regular follow-up visits are scheduled to monitor lung function and valve position. These visits usually occur at one week, one month, three months, and six months after the procedure.
Healthcare providers perform spirometry tests and physical examinations to evaluate improvement in breathing and overall lung performance. Any concerning symptoms, such as increasing shortness of breath or chest pain, require prompt medical attention.
Ongoing communication with the healthcare team is essential. Patients are also encouraged to participate in pulmonary rehabilitation and adhere to their prescribed medication regimen.
Clinical Evidence and Research
Multiple clinical trials have assessed the efficacy and safety of the Zephyr Valve in patients with severe emphysema. Studies such as the LIBERATE, TRANSFORM, and IMPACT trials have provided substantial data on patient outcomes.
The LIBERATE trial, a large multicenter randomized controlled study, showed that the Zephyr Valve improved lung function, exercise capacity, and quality of life for selected patients. At one year, patients reported significant improvements in forced expiratory volume (FEV1), six-minute walk distance (6MWD), and scores on health-related questionnaires.
The TRANSFORM and IMPACT studies reinforced these findings, indicating that the device can provide benefits for patients with heterogeneous and homogeneous emphysema. Both studies monitored patients for adverse events, with pneumothorax being the most commonly reported complication.
Key findings from these trials include:
- Significant improvement in FEV1 and 6MWD in treated patients versus controls.
- Reduction in hyperinflation measured by residual lung volume.
- Improved quality of life as assessed by validated questionnaires.
Note: Long-term follow-up data suggest sustained benefits in lung function and symptom control for a subset of patients. Ongoing research continues to evaluate durability of effects and identify which patient groups benefit most from the treatment.
Comparison With Other Lung Treatments
Zephyr Valve is a minimally invasive treatment primarily for severe emphysema. It is often compared to surgical lung volume reduction and other endobronchial valves regarding effectiveness, safety, and recovery time.
Zephyr Valve vs Surgery
Surgical lung volume reduction requires the removal of diseased lung tissue through an invasive procedure. This surgery typically involves general anesthesia, a hospital stay, and a longer recovery period compared to minimally invasive options.
The Zephyr Valve is placed through a bronchoscope and does not require any incisions. Most patients return home within a few days, and complications such as infection or prolonged air leaks are generally less frequent than with surgery.
While both methods can improve breathing and quality of life, surgery may provide greater benefits for some patients with specific lung damage patterns. The risk profiles differ, with surgery generally having higher rates of severe complications.
Zephyr Valve vs. Other Endobronchial Valves
The Zephyr Valve and other endobronchial valves, such as the Spiration Valve, share the goal of reducing lung hyperinflation by blocking airflow to damaged areas. Both devices are inserted via bronchoscopy and considered less invasive than surgery.
Key differences include valve design, size options, and specific eligibility criteria. The Zephyr Valve is one-way and self-expanding, designed for complete lobar occlusion. Some studies suggest similar short-term outcomes between devices, but long-term comparative data is limited.
Device selection may depend on the patient’s lung anatomy, physician experience, and reimbursement guidelines. Both are approved for severe emphysema but may differ in specific indications and contraindications.
Patient Candidacy and Evaluation
Patients considered for Zephyr Valve therapy typically have severe emphysema with significant hyperinflation that persists despite medication. Lung function tests are used to measure disease severity, focusing on parameters like FEV1, residual volume, and total lung capacity.
Key candidacy factors include:
- Diagnosis of severe heterogenous or homogeneous emphysema
- Evidence of air trapping and hyperinflation
- Symptoms that limit daily activities despite optimal medical management
A comprehensive clinical evaluation is essential. This includes a detailed medical history, physical examination, and assessment of prior treatments. Imaging studies, particularly high-resolution CT scans, are performed to map the areas of the lung most affected.
Certain conditions may exclude patients from treatment. Common exclusion criteria include significant collateral ventilation, active infections, recent exacerbations, and certain cardiac conditions. The presence of collateral ventilation is usually evaluated using specialized techniques to ensure effectiveness of the valve.
Multidisciplinary assessment is often recommended to determine suitability. Pulmonologists, radiologists, and respiratory therapists may all contribute to the evaluation process.
Note: Ongoing communication with the patient about expected outcomes, risks, and potential benefits is important throughout the decision-making process.
Long-Term Outcomes
Patients treated with the Zephyr Valve have shown sustained improvements in lung function over periods of up to five years. Studies report that benefits in forced expiratory volume (FEV1) and exercise capacity may persist for several years after the procedure.
Quality of life scores, including measures such as the St. George’s Respiratory Questionnaire, have also demonstrated lasting improvement. Patients often report less breathlessness and greater participation in daily activities post-procedure.
Serious complications are usually most likely to occur in the first months, but remain uncommon in the longer term. Some patients may require additional interventions, including valve removal or replacement.
Long-term monitoring and follow-up are recommended to track symptoms, lung function, and detect any late complications. Regular follow-up helps ensure optimal results and early intervention if issues arise.
The durability of response can vary. Some people maintain benefit for several years, while others experience a decline over time. Individual factors, such as severity of emphysema and comorbidities, influence these outcomes.
Medication needs may be reduced in some cases, but most patients continue with standard COPD therapies alongside valve treatment. Long-term data remains under evaluation as more patients receive the device and are followed for extended periods.
Accessibility and Insurance Coverage
The Zephyr Valve is available at many specialized healthcare centers throughout the United States and other countries. Access, however, often depends on healthcare provider expertise and the presence of an appropriate hospital or clinic.
Insurance coverage for the Zephyr Valve varies. Medicare, most Medicaid programs, and several private insurance plans may cover the procedure, but eligibility criteria apply. Patients typically need to meet specific diagnostic guidelines before approval.
Some common requirements for insurance coverage include:
- Documented diagnosis of severe emphysema or COPD
- Inadequate symptom control with medical therapy
- Evaluation by a multidisciplinary team
Out-of-pocket expenses can differ by plan, region, and pre-authorization approval. In some cases, patients may have to provide additional documentation or appeal coverage denials.
Prospective patients are encouraged to contact their insurance provider to confirm coverage. Many hospitals that offer the Zephyr Valve also have patient coordinators who help patients navigate the insurance process.
Note: Financial assistance programs may be available for uninsured or underinsured patients. Eligibility for these programs is determined by income and medical necessity.
FAQs About the Zephyr Valve
What Does a Zephyr Valve Do?
The Zephyr Valve is a small, one-way device placed in the airways of the lungs to treat severe emphysema, a type of COPD. Its main purpose is to block airflow into the most damaged areas of the lung, allowing trapped air to escape and the healthier parts of the lung to expand.
This improves breathing efficiency, reduces shortness of breath, and allows patients to be more physically active with less effort.
What Is the Success Rate of the Zephyr Valve?
Clinical studies have shown that the Zephyr Valve can lead to significant improvements in lung function, exercise tolerance, and quality of life in appropriately selected patients. Roughly 50–60% of patients who receive the valve report meaningful improvements.
However, success heavily depends on proper patient selection, especially the absence of collateral ventilation between lung lobes, which can reduce the valve’s effectiveness.
Who Is Not a Candidate for a Zephyr Valve?
Not all patients with COPD or emphysema are eligible for the Zephyr Valve. Those with significant collateral ventilation, active lung infections, or severe heart conditions may not be good candidates.
Additionally, patients who have had prior lung surgery or certain anatomical issues may not qualify. A detailed evaluation, including a CT scan and specialized testing, is required to determine eligibility.
What Are the Side Effects of the Zephyr Valve?
While the Zephyr Valve is generally well-tolerated, some patients may experience side effects or complications. Common side effects include short-term increased coughing, wheezing, or mucus production.
More serious risks can include pneumothorax (collapsed lung), pneumonia, or worsening of emphysema symptoms shortly after the procedure. Most side effects are treatable, but close monitoring after placement is important to manage potential issues promptly.
Does the Zephyr Valve Increase Life Expectancy?
The Zephyr Valve is designed to improve quality of life and lung function rather than directly extend life expectancy. However, by reducing lung hyperinflation and easing the work of breathing, the procedure may help reduce hospitalizations, increase physical activity, and slow functional decline.
Note: While more research is needed on long-term survival benefits, many patients report significant improvements in daily living and symptom control.
Are Zephyr Valves Permanent?
Zephyr Valves are intended to be a long-term solution but are not necessarily permanent. They are designed to remain in place and function for years, but they can be removed or repositioned if necessary.
In some cases, adjustments are made if complications arise or if the valve isn’t providing the expected benefit. The ability to reverse or modify the treatment adds to its safety and flexibility.
How Do Zephyr Valves Work?
Zephyr Valves are tiny one-way valves inserted into the airways of the lungs using a bronchoscope. They allow air and trapped gas to escape from the most damaged parts of the lung while preventing new air from entering those areas.
This process reduces lung hyperinflation, allowing the healthier parts of the lung to expand and function more effectively. As a result, patients can breathe more easily and experience reduced shortness of breath.
Is the Zephyr Valve Available in Canada?
Yes, the Zephyr Valve is available in Canada. It has been approved for use in several countries, including Canada, for patients with severe emphysema. However, availability may vary by province, hospital system, and physician training.
Patients interested in the procedure should speak with a pulmonologist or respiratory specialist to find out if they qualify and where the procedure is offered in their area.
How Much Does a Zephyr Valve Cost?
The total cost of Zephyr Valve treatment can range from $15,000 to $30,000 or more, depending on the healthcare provider, location, and number of valves needed. This includes not just the cost of the devices but also hospital fees, imaging, and physician services.
Note: Insurance coverage can significantly reduce out-of-pocket costs, so patients should verify their benefits and consult their provider for a personalized cost estimate.
Are Zephyr Valves Covered by Medicare?
Yes, Zephyr Valves are covered by Medicare for eligible patients with severe emphysema. Coverage typically includes the procedure, the devices, and related hospital services, provided that the patient meets specific clinical criteria. These include confirmed diagnosis, absence of collateral ventilation, and certain lung function test results.
However, coverage details may vary by region and provider, so it’s important for patients to consult with their pulmonologist and Medicare representative to ensure eligibility and understand any potential out-of-pocket costs.
Final Thoughts
The Zephyr Valve offers a new sense of hope for patients with advanced emphysema who no longer find relief from medications or pulmonary rehabilitation alone.
By helping reduce lung hyperinflation, the valve enables better breathing, improved exercise capacity, and enhanced quality of life. Though not a cure, it serves as a meaningful intervention for selected patients who meet the criteria.
As with any treatment, understanding how it works and discussing the risks and benefits with a healthcare provider is key. With innovations like the Zephyr Valve, managing COPD is becoming more effective and personalized than ever before.
Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
References
- Pahal P, Avula A, Sharma S. Emphysema. [Updated 2023 Jan 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Zephyr Valve Treatment for COPD and Emphysema | Michigan Medicine. 2025.
- Lovering, Cathy. “What You Need to Know About the Zephyr Valve for COPD.” Healthline, 12 Oct. 2022.
- Dransfield, Mark T., et al. “Effect of Zephyr Endobronchial Valves on Dyspnea, Activity Levels, and Quality of Life at One Year. Results From a Randomized Clinical Trial.” Annals of the American Thoracic Society, vol. 17, no. 7, American Thoracic Society, Mar. 2020.
- Iavarone, Stefano. “What to Know About Zephyr Valve Treatment: How It Works.” www.medicalnewstoday.com, Jan. 2022.
- Pulmonx Corporation. “Pulmonx | Patient Selection Criteria for Zephyr Endobronchial Valves.” Pulmonx Corporation, 1 Feb. 2023.


